Neuronal activity biases axon selection for myelination in vivo
- PMID: 25849987
- PMCID: PMC4414883
- DOI: 10.1038/nn.3992
Neuronal activity biases axon selection for myelination in vivo
Abstract
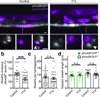
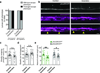
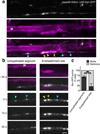
An essential feature of vertebrate neural development is ensheathment of axons with myelin, an insulating membrane formed by oligodendrocytes. Not all axons are myelinated, but mechanisms directing myelination of specific axons are unknown. Using zebrafish, we found that activity-dependent secretion stabilized myelin sheath formation on select axons. When VAMP2-dependent exocytosis was silenced in single axons, oligodendrocytes preferentially ensheathed neighboring axons. Nascent sheaths formed on silenced axons were shorter in length, but when activity of neighboring axons was also suppressed, inhibition of sheath growth was relieved. Using in vivo time-lapse microscopy, we found that only 25% of oligodendrocyte processes that initiated axon wrapping were stabilized during normal development and that initiation did not require activity. Instead, oligodendrocyte processes wrapping silenced axons retracted more frequently. We propose that axon selection for myelination results from excessive and indiscriminate initiation of wrapping followed by refinement that is biased by activity-dependent secretion from axons.
Figures



Comment in
-
Myelination: An active process.Nat Rev Neurosci. 2015 Jun;16(6):314-5. doi: 10.1038/nrn3964. Epub 2015 Apr 29. Nat Rev Neurosci. 2015. PMID: 25921814 No abstract available.
Similar articles
-
Individual neuronal subtypes control initial myelin sheath growth and stabilization.Neural Dev. 2020 Sep 28;15(1):12. doi: 10.1186/s13064-020-00149-3. Neural Dev. 2020. PMID: 32988384 Free PMC article.
-
In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development.Nat Neurosci. 2006 Dec;9(12):1506-11. doi: 10.1038/nn1803. Epub 2006 Nov 12. Nat Neurosci. 2006. PMID: 17099706
-
Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis.Dev Neurosci. 1996;18(4):243-54. doi: 10.1159/000111414. Dev Neurosci. 1996. PMID: 8911764
-
Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum.J Neurosci Res. 2000 Feb 15;59(4):477-88. doi: 10.1002/(SICI)1097-4547(20000215)59:4<477::AID-JNR2>3.0.CO;2-J. J Neurosci Res. 2000. PMID: 10679786 Review.
-
Mechanisms regulating the development of oligodendrocytes and central nervous system myelin.Neuroscience. 2014 Sep 12;276:29-47. doi: 10.1016/j.neuroscience.2013.11.029. Epub 2013 Nov 22. Neuroscience. 2014. PMID: 24275321 Review.
Cited by
-
Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity.J Neurosci. 2016 Jun 29;36(26):6937-48. doi: 10.1523/JNEUROSCI.0908-16.2016. J Neurosci. 2016. PMID: 27358452 Free PMC article.
-
Heterogeneity and regulation of oligodendrocyte morphology.Front Cell Dev Biol. 2022 Oct 24;10:1030486. doi: 10.3389/fcell.2022.1030486. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393856 Free PMC article. Review.
-
Presentation and integration of multiple signals that modulate oligodendrocyte lineage progression and myelination.Front Cell Neurosci. 2022 Nov 14;16:1041853. doi: 10.3389/fncel.2022.1041853. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36451655 Free PMC article. Review.
-
Oligodendrocyte Development in the Absence of Their Target Axons In Vivo.PLoS One. 2016 Oct 7;11(10):e0164432. doi: 10.1371/journal.pone.0164432. eCollection 2016. PLoS One. 2016. PMID: 27716830 Free PMC article.
-
DETECTION AND TRACKING OF MIGRATING OLIGODENDROCYTE PROGENITOR CELLS FROM IN VIVO FLUORESCENCE TIME-LAPSE IMAGING DATA.Proc IEEE Int Symp Biomed Imaging. 2018 Apr;2018:961-964. doi: 10.1109/ISBI.2018.8363730. Epub 2018 May 24. Proc IEEE Int Symp Biomed Imaging. 2018. PMID: 30598726 Free PMC article.
References
-
- Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 1980;6:415–420. - PubMed
-
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–266. - PubMed
-
- Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

