High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability
- PMID: 25837594
- PMCID: PMC4383570
- DOI: 10.1371/journal.pone.0122195
High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability
Abstract
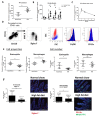
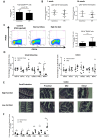
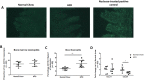
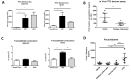
The development of intestinal permeability and the penetration of microbial products are key factors associated with the onset of metabolic disease. However, the mechanisms underlying this remain unclear. Here we show that, unlike liver or adipose tissue, high fat diet (HFD)/obesity in mice does not cause monocyte/macrophage infiltration into the intestine or pro-inflammatory changes in gene expression. Rather HFD causes depletion of intestinal eosinophils associated with the onset of intestinal permeability. Intestinal eosinophil numbers were restored by returning HFD fed mice to normal chow and were unchanged in leptin-deficient (Ob/Ob) mice, indicating that eosinophil depletion is caused specifically by a high fat diet and not obesity per se. Analysis of different aspects of intestinal permeability in HFD fed and Ob/Ob mice shows an association between eosinophil depletion and ileal paracelullar permeability, as well as leakage of albumin into the feces, but not overall permeability to FITC dextran. These findings provide the first evidence that a high fat diet causes intestinal eosinophil depletion, rather than inflammation, which may contribute to defective barrier integrity and the onset of metabolic disease.
Conflict of interest statement
Figures

Similar articles
-
Disturbed intestinal nitrogen homeostasis in a mouse model of high-fat diet-induced obesity and glucose intolerance.Am J Physiol Endocrinol Metab. 2014 Mar;306(6):E668-80. doi: 10.1152/ajpendo.00437.2013. Epub 2014 Jan 14. Am J Physiol Endocrinol Metab. 2014. PMID: 24425764
-
Lactobacillus gasseri SBT2055 inhibits adipose tissue inflammation and intestinal permeability in mice fed a high-fat diet.J Nutr Sci. 2016 May 30;5:e23. doi: 10.1017/jns.2016.12. eCollection 2016. J Nutr Sci. 2016. PMID: 27293560 Free PMC article.
-
Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice.Endocrinology. 2014 Jul;155(7):2411-22. doi: 10.1210/en.2013-1861. Epub 2014 Apr 17. Endocrinology. 2014. PMID: 24742194
-
Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice.Obesity (Silver Spring). 2015 Jul;23(7):1429-39. doi: 10.1002/oby.21122. Epub 2015 Jun 5. Obesity (Silver Spring). 2015. PMID: 26053244
-
High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice.Aging (Albany NY). 2015 Apr;7(4):256-68. doi: 10.18632/aging.100738. Aging (Albany NY). 2015. PMID: 25979814 Free PMC article.
Cited by
-
Nutrient-derived signals regulate eosinophil adaptation to the small intestine.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2316446121. doi: 10.1073/pnas.2316446121. Epub 2024 Jan 25. Proc Natl Acad Sci U S A. 2024. PMID: 38271336 Free PMC article.
-
The aryl hydrocarbon receptor contributes to tissue adaptation of intestinal eosinophils in mice.J Exp Med. 2022 Apr 4;219(4):e20210970. doi: 10.1084/jem.20210970. Epub 2022 Mar 3. J Exp Med. 2022. PMID: 35238865 Free PMC article.
-
Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction.Mol Metab. 2022 Mar;57:101438. doi: 10.1016/j.molmet.2022.101438. Epub 2022 Jan 8. Mol Metab. 2022. PMID: 35007789 Free PMC article.
-
Hepatic Immune Microenvironment in Alcoholic and Nonalcoholic Liver Disease.Biomed Res Int. 2017;2017:6862439. doi: 10.1155/2017/6862439. Epub 2017 Aug 9. Biomed Res Int. 2017. PMID: 28852648 Free PMC article. Review.
-
Distinct Hepatic Macrophage Populations in Lean and Obese Mice.Front Endocrinol (Lausanne). 2016 Dec 6;7:152. doi: 10.3389/fendo.2016.00152. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27999564 Free PMC article. Review.
References
-
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011; 3: 559–572. 10.1002/emmm.201100159 - DOI - PMC - PubMed
-
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007; 56: 1761–1772. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

