Soluble Urokinase Receptor Is Released Selectively by Glioblastoma Cells That Express Epidermal Growth Factor Receptor Variant III and Promotes Tumor Cell Migration and Invasion
- PMID: 25837250
- PMCID: PMC4463428
- DOI: 10.1074/jbc.M115.637488
Soluble Urokinase Receptor Is Released Selectively by Glioblastoma Cells That Express Epidermal Growth Factor Receptor Variant III and Promotes Tumor Cell Migration and Invasion
Abstract
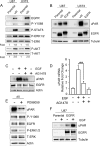
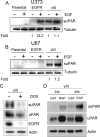
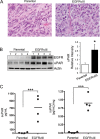
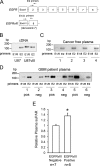
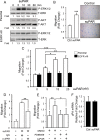
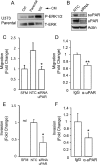
Genomic heterogeneity is characteristic of glioblastoma (GBM). In many GBMs, the EGF receptor gene (EGFR) is amplified and may be truncated to generate a constitutively active form of the receptor called EGFRvIII. EGFR gene amplification and EGFRvIII are associated with GBM progression, even when only a small fraction of the tumor cells express EGFRvIII. In this study, we show that EGFRvIII-positive GBM cells express significantly increased levels of cellular urokinase receptor (uPAR) and release increased amounts of soluble uPAR (suPAR). When mice were xenografted with human EGFRvIII-expressing GBM cells, tumor-derived suPAR was detected in the plasma, and the level was significantly increased compared with that detected in plasma samples from control mice xenografted with EGFRvIII-negative GBM cells. suPAR also was increased in plasma from patients with EGFRvIII-positive GBMs. Purified suPAR was biologically active when added to cultures of EGFRvIII-negative GBM cells, activating cell signaling and promoting cell migration and invasion. suPAR did not significantly stimulate cell signaling or migration of EGFRvIII-positive cells, probably because cell signaling was already substantially activated in these cells. The activities of suPAR were replicated by conditioned medium (CM) from EGFRvIII-positive GBM cells. When the CM was preincubated with uPAR-neutralizing antibody or when uPAR gene expression was silenced in cells used to prepare CM, the activity of the CM was significantly attenuated. These results suggest that suPAR may function as an important paracrine signaling factor in EGFRvIII-positive GBMs, inducing an aggressive phenotype in tumor cells that are EGFRvIII-negative.
Keywords: EGFRvIII; LRP1; cell invasion; cell migration; epidermal growth factor receptor (EGFR); glioblastoma; paracrine interaction; paracrine signaling; tumor microenvironment; urokinase receptor.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Selective coexpression of VEGF receptor 2 in EGFRvIII-positive glioblastoma cells prevents cellular senescence and contributes to their aggressive nature.Neuro Oncol. 2016 May;18(5):667-78. doi: 10.1093/neuonc/nov243. Epub 2015 Sep 29. Neuro Oncol. 2016. PMID: 26420897 Free PMC article.
-
Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells.Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15984-9. doi: 10.1073/pnas.1113416108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896743 Free PMC article.
-
Neutralizing the EGF receptor in glioblastoma cells stimulates cell migration by activating uPAR-initiated cell signaling.Oncogene. 2015 Jul 30;34(31):4078-88. doi: 10.1038/onc.2014.336. Epub 2014 Oct 27. Oncogene. 2015. PMID: 25347738 Free PMC article.
-
The Role of EGFR-Met Interactions in the Pathogenesis of Glioblastoma and Resistance to Treatment.Curr Cancer Drug Targets. 2017;17(3):297-302. doi: 10.2174/1568009616666161215162515. Curr Cancer Drug Targets. 2017. PMID: 28004613 Review.
-
The EGFRvIII variant in glioblastoma multiforme.J Clin Neurosci. 2009 Jun;16(6):748-54. doi: 10.1016/j.jocn.2008.12.005. Epub 2009 Mar 25. J Clin Neurosci. 2009. PMID: 19324552 Review.
Cited by
-
Expression and activity of the urokinase plasminogen activator system in canine primary brain tumors.Onco Targets Ther. 2017 Apr 12;10:2077-2085. doi: 10.2147/OTT.S132964. eCollection 2017. Onco Targets Ther. 2017. PMID: 28442916 Free PMC article.
-
The Unwanted Cell Migration in the Brain: Glioma Metastasis.Neurochem Res. 2017 Jun;42(6):1847-1863. doi: 10.1007/s11064-017-2272-2. Epub 2017 May 6. Neurochem Res. 2017. PMID: 28478595 Review.
-
Selective coexpression of VEGF receptor 2 in EGFRvIII-positive glioblastoma cells prevents cellular senescence and contributes to their aggressive nature.Neuro Oncol. 2016 May;18(5):667-78. doi: 10.1093/neuonc/nov243. Epub 2015 Sep 29. Neuro Oncol. 2016. PMID: 26420897 Free PMC article.
-
Cleavage of the urokinase receptor (uPAR) on oral cancer cells: regulation by transforming growth factor - β1 (TGF-β1) and potential effects on migration and invasion.BMC Cancer. 2017 May 19;17(1):350. doi: 10.1186/s12885-017-3349-7. BMC Cancer. 2017. PMID: 28526008 Free PMC article.
-
Plasminogen activator receptor assemblies in cell signaling, innate immunity, and inflammation.Am J Physiol Cell Physiol. 2021 Oct 1;321(4):C721-C734. doi: 10.1152/ajpcell.00269.2021. Epub 2021 Aug 18. Am J Physiol Cell Physiol. 2021. PMID: 34406905 Free PMC article. Review.
References
-
- Smith H. W., Marshall C. J. (2010) Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 11, 23–36 - PubMed
-
- Collen D. (1999) The plasminogen (fibrinolytic) system. Thromb. Haemost. 82, 259–270 - PubMed
-
- Liu D., Aguirre Ghiso J., Estrada Y., Ossowski L. (2002) EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1, 445–457 - PubMed
-
- Wei Y., Lukashev M., Simon D. I., Bodary S. C., Rosenberg S., Doyle M. V., Chapman H. A. (1996) Regulation of integrin function by the urokinase receptor. Science 273, 1551–1555 - PubMed
-
- Tarui T., Mazar A. P., Cines D. B., Takada Y. (2001) Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J. Biol. Chem. 276, 3983–3990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

