Detection of Simian Immunodeficiency Virus in Semen, Urethra, and Male Reproductive Organs during Efficient Highly Active Antiretroviral Therapy
- PMID: 25833047
- PMCID: PMC4442442
- DOI: 10.1128/JVI.03628-14
Detection of Simian Immunodeficiency Virus in Semen, Urethra, and Male Reproductive Organs during Efficient Highly Active Antiretroviral Therapy
Abstract
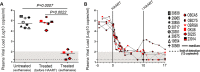
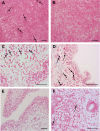
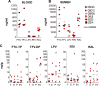
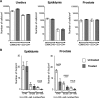
A number of men receiving prolonged suppressive highly active antiretroviral therapy (HAART) still shed human immunodeficiency virus (HIV) in semen. To investigate whether this seminal shedding may be due to poor drug penetration and/or viral production by long-lived cells within male genital tissues, we analyzed semen and reproductive tissues from macaques chronically infected with simian immunodeficiency virus mac251 (SIVmac251) who were treated for 4 months with HAART, which was intensified over the last 7 weeks with an integrase inhibitor. We showed that a subset of treated animals continued shedding SIV in semen despite efficient HAART. This shedding was not associated with low antiretroviral drug concentrations in semen or in testis, epididymis, seminal vesicles, and prostate. HAART had no significant impact on SIV RNA in the urethra, whereas it drastically reduced SIV RNA levels in the prostate and vas deferens and to a lesser extent in the epididymis and seminal vesicle. The only detectable SIV RNA-positive cells within the male genital tract after HAART were urethral macrophages. SIV DNA levels in genital tissues were not decreased by HAART, suggesting the presence throughout the male genital tract of nonproductively infected cells. In conclusion, our results demonstrate that 4 months of HAART induced variable and limited control of viral infection in the male reproductive organs, particularly in the urethra, and suggest that infected long-lived cells in the male genital tract may be involved in persistent seminal shedding during HAART. These results pave the way for further investigations of male genital organ infection in long-term-treated infected individuals.
Importance: A substantial subset of men receiving prolonged HAART suppressing viral loads in the blood still harbor HIV in semen, and cases of sexual transmission have been reported. To understand the origin of this persistence, we analyzed the semen and male reproductive tissues from SIV-infected macaques treated with HAART. We demonstrated that persistent seminal shedding was not linked to poor drug penetration in semen or semen-producing prostate, seminal vesicle, epididymis, and testis. We revealed that HAART decreased SIV RNA to various extents in all male genital organs, with the exception of the urethra, in which SIV RNA(+) macrophages were observed despite HAART. Importantly, HAART did not impact SIV DNA levels in the male genital organs. These results suggest that infection of male genital organs, and particularly the urethra, could be involved in the release of virus in semen during HAART.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Seminal Simian Immunodeficiency Virus in Chronically Infected Cynomolgus Macaques Is Dominated by Virus Originating from Multiple Genital Organs.J Virol. 2018 Jun 29;92(14):e00133-18. doi: 10.1128/JVI.00133-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29720516 Free PMC article.
-
Impact of short-term HAART initiated during the chronic stage or shortly post-exposure on SIV infection of male genital organs.PLoS One. 2012;7(5):e37348. doi: 10.1371/journal.pone.0037348. Epub 2012 May 17. PLoS One. 2012. PMID: 22615988 Free PMC article.
-
Viral RNA levels and env variants in semen and tissues of mature male rhesus macaques infected with SIV by penile inoculation.PLoS One. 2013 Oct 11;8(10):e76367. doi: 10.1371/journal.pone.0076367. eCollection 2013. PLoS One. 2013. PMID: 24146859 Free PMC article.
-
[The male genital tract: A host for HIV].Gynecol Obstet Fertil. 2007 Dec;35(12):1245-50. doi: 10.1016/j.gyobfe.2007.09.017. Epub 2007 Nov 26. Gynecol Obstet Fertil. 2007. PMID: 18035579 Review. French.
-
Origins of HIV-infected leukocytes and virions in semen.J Infect Dis. 2014 Dec 15;210 Suppl 3:S622-30. doi: 10.1093/infdis/jiu328. J Infect Dis. 2014. PMID: 25414416 Review.
Cited by
-
HIV Latency in Myeloid Cells: Challenges for a Cure.Pathogens. 2022 May 24;11(6):611. doi: 10.3390/pathogens11060611. Pathogens. 2022. PMID: 35745465 Free PMC article. Review.
-
More than a Gender Issue: Testis as a Distinctive HIV Reservoir and Its Implication for Viral Eradication.Methods Mol Biol. 2022;2407:173-186. doi: 10.1007/978-1-0716-1871-4_13. Methods Mol Biol. 2022. PMID: 34985665
-
Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection.PLoS Pathog. 2015 Sep 24;11(9):e1005153. doi: 10.1371/journal.ppat.1005153. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26402858 Free PMC article.
-
HIV Diversity and Genetic Compartmentalization in Blood and Testes during Suppressive Antiretroviral Therapy.J Virol. 2019 Aug 13;93(17):e00755-19. doi: 10.1128/JVI.00755-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189714 Free PMC article.
-
Histomorphometric changes in testis following administration of tenofovir nanoparticles in an animal model.Discov Nano. 2024 Mar 25;19(1):56. doi: 10.1186/s11671-024-04002-y. Discov Nano. 2024. PMID: 38526666 Free PMC article.
References
-
- UNAIDS. 2013. Report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland.
-
- Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, Mugo NR, Wald A, Corey L, Donnell D, Campbell MS, Mullins JI, Celum C. 2011. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 3:77ra29. doi:10.1126/scitranslmed.3001888. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

