Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo
- PMID: 25817203
- PMCID: PMC4415852
- DOI: 10.1016/j.ccell.2015.02.016
Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo
Abstract
Chromosomal translocations affecting mixed lineage leukemia gene (MLL) result in acute leukemias resistant to therapy. The leukemogenic activity of MLL fusion proteins is dependent on their interaction with menin, providing basis for therapeutic intervention. Here we report the development of highly potent and orally bioavailable small-molecule inhibitors of the menin-MLL interaction, MI-463 and MI-503, and show their profound effects in MLL leukemia cells and substantial survival benefit in mouse models of MLL leukemia. Finally, we demonstrate the efficacy of these compounds in primary samples derived from MLL leukemia patients. Overall, we demonstrate that pharmacologic inhibition of the menin-MLL interaction represents an effective treatment for MLL leukemias in vivo and provide advanced molecular scaffold for clinical lead identification.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no relevant conflict of interest.
Figures

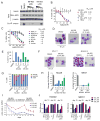
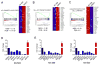
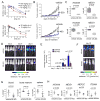

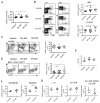

Comment in
-
Designed to kill: novel menin-MLL inhibitors target MLL-rearranged leukemia.Cancer Cell. 2015 Apr 13;27(4):431-3. doi: 10.1016/j.ccell.2015.03.012. Cancer Cell. 2015. PMID: 25873167
-
Therapy: It's raining menin.Nat Rev Cancer. 2015 May;15(5):256-7. doi: 10.1038/nrc3951. Epub 2015 Apr 16. Nat Rev Cancer. 2015. PMID: 25877330 No abstract available.
Similar articles
-
Co-inhibition of HDAC and MLL-menin interaction targets MLL-rearranged acute myeloid leukemia cells via disruption of DNA damage checkpoint and DNA repair.Clin Epigenetics. 2019 Oct 7;11(1):137. doi: 10.1186/s13148-019-0723-0. Clin Epigenetics. 2019. PMID: 31590682 Free PMC article.
-
Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia.Blood. 2012 Nov 29;120(23):4461-9. doi: 10.1182/blood-2012-05-429274. Epub 2012 Aug 30. Blood. 2012. PMID: 22936661 Free PMC article.
-
Molecular basis of the mixed lineage leukemia-menin interaction: implications for targeting mixed lineage leukemias.J Biol Chem. 2010 Dec 24;285(52):40690-8. doi: 10.1074/jbc.M110.172783. Epub 2010 Oct 20. J Biol Chem. 2010. PMID: 20961854 Free PMC article.
-
A Box of Chemistry to Inhibit the MEN1 Tumor Suppressor Gene Promoting Leukemia.ChemMedChem. 2021 May 6;16(9):1391-1402. doi: 10.1002/cmdc.202000972. Epub 2021 Mar 10. ChemMedChem. 2021. PMID: 33534953 Free PMC article. Review.
-
Recent Progress of Small Molecule Menin-MLL Interaction Inhibitors as Therapeutic Agents for Acute Leukemia.J Med Chem. 2021 Nov 11;64(21):15519-15533. doi: 10.1021/acs.jmedchem.1c00872. Epub 2021 Nov 2. J Med Chem. 2021. PMID: 34726905 Review.
Cited by
-
Systematic characterization of the HOXA9 downstream targets in MLL-r leukemia by noncoding CRISPR screens.Nat Commun. 2023 Nov 28;14(1):7464. doi: 10.1038/s41467-023-43264-5. Nat Commun. 2023. PMID: 38016946 Free PMC article.
-
Small-Molecule Inhibition of the Acyl-Lysine Reader ENL as a Strategy against Acute Myeloid Leukemia.Cancer Discov. 2022 Nov 2;12(11):2684-2709. doi: 10.1158/2159-8290.CD-21-1307. Cancer Discov. 2022. PMID: 36053276 Free PMC article.
-
Towards precision medicine for AML.Nat Rev Clin Oncol. 2021 Sep;18(9):577-590. doi: 10.1038/s41571-021-00509-w. Epub 2021 May 18. Nat Rev Clin Oncol. 2021. PMID: 34006997 Review.
-
Menin-regulated Pbk controls high fat diet-induced compensatory beta cell proliferation.EMBO Mol Med. 2021 May 7;13(5):e13524. doi: 10.15252/emmm.202013524. Epub 2021 Apr 6. EMBO Mol Med. 2021. PMID: 33821572 Free PMC article.
-
Therapeutic targeting in pediatric acute myeloid leukemia with aberrant HOX/MEIS1 expression.Eur J Med Genet. 2023 Dec;66(12):104869. doi: 10.1016/j.ejmg.2023.104869. Epub 2023 Oct 29. Eur J Med Genet. 2023. PMID: 38174649 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

