Minor Structural Change to Tertiary Sulfonamide RORc Ligands Led to Opposite Mechanisms of Action
- PMID: 25815138
- PMCID: PMC4360161
- DOI: 10.1021/ml500420y
Minor Structural Change to Tertiary Sulfonamide RORc Ligands Led to Opposite Mechanisms of Action
Abstract
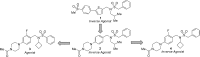
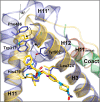
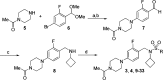
A minor structural change to tertiary sulfonamide RORc ligands led to distinct mechanisms of action. Co-crystal structures of two compounds revealed mechanistically consistent protein conformational changes. Optimized phenylsulfonamides were identified as RORc agonists while benzylsulfonamides exhibited potent inverse agonist activity. Compounds behaving as agonists in our biochemical assay also gave rise to an increased production of IL-17 in human PBMCs whereas inverse agonists led to significant suppression of IL-17 under the same assay conditions. The most potent inverse agonist compound showed >180-fold selectivity over the ROR isoforms as well as all other nuclear receptors that were profiled.
Keywords: IL-17; PBMC; RORc; RORγ; TH17; agonist; inverse agonist.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Identification of tertiary sulfonamides as RORc inverse agonists.Bioorg Med Chem Lett. 2014 May 1;24(9):2182-7. doi: 10.1016/j.bmcl.2014.03.038. Epub 2014 Mar 21. Bioorg Med Chem Lett. 2014. PMID: 24685544
-
Discovery of 1-{4-[3-fluoro-4-((3s,6r)-3-methyl-1,1-dioxo-6-phenyl-[1,2]thiazinan-2-ylmethyl)-phenyl]-piperazin-1-yl}-ethanone (GNE-3500): a potent, selective, and orally bioavailable retinoic acid receptor-related orphan receptor C (RORc or RORγ) inverse agonist.J Med Chem. 2015 Jul 9;58(13):5308-22. doi: 10.1021/acs.jmedchem.5b00597. Epub 2015 Jun 23. J Med Chem. 2015. PMID: 26061388
-
Discovery of oxa-sultams as RORc inverse agonists showing reduced lipophilicity, improved selectivity and favorable ADME properties.Bioorg Med Chem Lett. 2016 Sep 15;26(18):4455-4461. doi: 10.1016/j.bmcl.2016.07.081. Epub 2016 Aug 3. Bioorg Med Chem Lett. 2016. PMID: 27524313
-
Identification of a novel selective inverse agonist probe and analogs for the Retinoic acid receptor-related Orphan Receptor Gamma (RORγ).2012 Apr 16 [updated 2013 Mar 14]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2012 Apr 16 [updated 2013 Mar 14]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23762937 Free Books & Documents. Review.
-
RORγ Structural Plasticity and Druggability.Int J Mol Sci. 2020 Jul 27;21(15):5329. doi: 10.3390/ijms21155329. Int J Mol Sci. 2020. PMID: 32727079 Free PMC article. Review.
Cited by
-
Switch in Site of Inhibition: A Strategy for Structure-Based Discovery of Human Topoisomerase IIα Catalytic Inhibitors.ACS Med Chem Lett. 2015 Feb 23;6(4):481-5. doi: 10.1021/acsmedchemlett.5b00040. eCollection 2015 Apr 9. ACS Med Chem Lett. 2015. PMID: 25941559 Free PMC article.
-
The application of machine learning methods to the prediction of novel ligands for RORγ/RORγT receptors.Comput Struct Biotechnol J. 2023 Oct 29;21:5491-5505. doi: 10.1016/j.csbj.2023.10.021. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 38022699 Free PMC article.
-
Targeting the cholesterol-RORα/γ axis inhibits colorectal cancer progression through degrading c-myc.Oncogene. 2022 Dec;41(49):5266-5278. doi: 10.1038/s41388-022-02515-3. Epub 2022 Oct 31. Oncogene. 2022. PMID: 36316442 Free PMC article.
-
(Inverse) Agonists of Retinoic Acid-Related Orphan Receptor γ: Regulation of Immune Responses, Inflammation, and Autoimmune Disease.Annu Rev Pharmacol Toxicol. 2020 Jan 6;60:371-390. doi: 10.1146/annurev-pharmtox-010919-023711. Epub 2019 Aug 6. Annu Rev Pharmacol Toxicol. 2020. PMID: 31386594 Free PMC article. Review.
-
Structural change of retinoic-acid receptor-related orphan receptor induced by binding of inverse-agonist: Molecular dynamics and ab initio molecular orbital simulations.Comput Struct Biotechnol J. 2020 Jun 25;18:1676-1685. doi: 10.1016/j.csbj.2020.06.034. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32670507 Free PMC article.
References
-
- Hirose T.; Smith R. J.; Jetten A. Biochem. Biophys. Res. Commun. 1994, 205, 1976. - PubMed
-
- Ivanov I. I.; McKenzie B. S.; Zhou L.; Takodoro C. E.; Lepelley A.; Lafaille J. J.; Cua D. J.; Littman D. R. Cell 2006, 126, 1121. - PubMed
-
- Sabat R.; Ouyang W.; Wolk K. Nat. Rev. Drug Disc. 2014, 13, 21. - PubMed
-
- Codarri L.; Gyülvészi G.; Tosevski V.; Hesske L.; Fontana A.; Magnenat L.; Suter T.; Becher B. Nat. Immunol. 2011, 12, 560. - PubMed
-
- Rich P.; Sigurgeirsson B.; Thaci D.; Ortonne J.-P.; Paul C.; Schopf R. E.; Morita A.; Roseau K.; Harfst E.; Guettner A.; Machacek M.; Papavassilis C. Br. J. Dermatol. 2013, 168, 402. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

