Switch-like responses of two cholesterol sensors do not require protein oligomerization in membranes
- PMID: 25809258
- PMCID: PMC4375629
- DOI: 10.1016/j.bpj.2015.02.008
Switch-like responses of two cholesterol sensors do not require protein oligomerization in membranes
Abstract
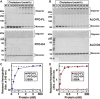
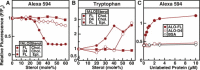
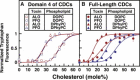
Many cellular processes are sensitive to levels of cholesterol in specific membranes and show a strongly sigmoidal dependence on membrane composition. The sigmoidal responses of the cholesterol sensors involved in these processes could arise from several mechanisms, including positive cooperativity (protein effects) and limited cholesterol accessibility (membrane effects). Here, we describe a sigmoidal response that arises primarily from membrane effects due to sharp changes in the chemical activity of cholesterol. Our models for eukaryotic membrane-bound cholesterol sensors are soluble bacterial toxins that show an identical switch-like specificity for endoplasmic reticulum membrane cholesterol. We show that truncated versions of these toxins fail to form oligomers but still show sigmoidal binding to cholesterol-containing membranes. The nonlinear response emerges because interactions between bilayer lipids control cholesterol accessibility to toxins in a threshold-like fashion. Around these thresholds, the affinity of toxins for membrane cholesterol varies by >100-fold, generating highly cooperative lipid-dependent responses independently of protein-protein interactions. Such lipid-driven cooperativity may control the sensitivity of many cholesterol-dependent processes.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Accessibility of cholesterol in endoplasmic reticulum membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold.J Biol Chem. 2010 Sep 17;285(38):29480-90. doi: 10.1074/jbc.M110.148254. Epub 2010 Jun 23. J Biol Chem. 2010. PMID: 20573965 Free PMC article.
-
Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit beta-strand alignment.Nat Struct Mol Biol. 2004 Aug;11(8):697-705. doi: 10.1038/nsmb793. Epub 2004 Jul 4. Nat Struct Mol Biol. 2004. PMID: 15235590
-
Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin.Nat Struct Biol. 2002 Nov;9(11):823-7. doi: 10.1038/nsb855. Nat Struct Biol. 2002. PMID: 12368903
-
Perfringolysin O structure and mechanism of pore formation as a paradigm for cholesterol-dependent cytolysins.Subcell Biochem. 2014;80:63-81. doi: 10.1007/978-94-017-8881-6_5. Subcell Biochem. 2014. PMID: 24798008 Free PMC article. Review.
-
Cholesterol-Dependent Cytolysins: Membrane and Protein Structural Requirements for Pore Formation.Chem Rev. 2019 Jul 10;119(13):7721-7736. doi: 10.1021/acs.chemrev.9b00090. Epub 2019 Jun 17. Chem Rev. 2019. PMID: 31244002 Review.
Cited by
-
Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol.EMBO J. 2020 Nov 2;39(21):e106057. doi: 10.15252/embj.2020106057. Epub 2020 Oct 5. EMBO J. 2020. PMID: 32944968 Free PMC article.
-
25-Hydroxycholesterol in health and diseases.J Lipid Res. 2024 Jan;65(1):100486. doi: 10.1016/j.jlr.2023.100486. Epub 2023 Dec 16. J Lipid Res. 2024. PMID: 38104944 Free PMC article. Review.
-
A concerted mechanism involving ACAT and SREBPs by which oxysterols deplete accessible cholesterol to restrict microbial infection.Elife. 2023 Jan 25;12:e83534. doi: 10.7554/eLife.83534. Elife. 2023. PMID: 36695568 Free PMC article.
-
The Chemical Potential of Plasma Membrane Cholesterol: Implications for Cell Biology.Biophys J. 2018 Feb 27;114(4):904-918. doi: 10.1016/j.bpj.2017.12.042. Biophys J. 2018. PMID: 29490250 Free PMC article.
-
Cholesterol access in cellular membranes controls Hedgehog signaling.Nat Chem Biol. 2020 Dec;16(12):1303-1313. doi: 10.1038/s41589-020-00678-2. Epub 2020 Nov 16. Nat Chem Biol. 2020. PMID: 33199907 Free PMC article. Review.
References
-
- Brown M.S., Goldstein J.L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

