Inhaled corticosteroids: potency, dose equivalence and therapeutic index
- PMID: 25808113
- PMCID: PMC4574823
- DOI: 10.1111/bcp.12637
Inhaled corticosteroids: potency, dose equivalence and therapeutic index
Abstract
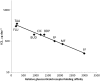
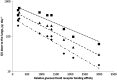
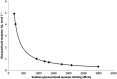
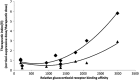
Glucocorticosteroids are a group of structurally related molecules that includes natural hormones and synthetic drugs with a wide range of anti-inflammatory potencies. For synthetic corticosteroid analogues it is commonly assumed that the therapeutic index cannot be improved by increasing their glucocorticoid receptor binding affinity. The validity of this assumption, particularly for inhaled corticosteroids, has not been fully explored. Inhaled corticosteroids exert their anti-inflammatory activity locally in the airways, and hence this can be dissociated from their potential to cause systemic adverse effects. The molecular structural features that increase glucocorticoid receptor binding affinity and selectivity drive topical anti-inflammatory activity. However, in addition, these structural modifications also result in physicochemical and pharmacokinetic changes that can enhance targeting to the airways and reduce systemic exposure. As a consequence, potency and therapeutic index can be correlated. However, this consideration is not reflected in asthma treatment guidelines that classify inhaled corticosteroid formulations as low-, mid- and high dose, and imbed a simple dose equivalence approach where potency is not considered to affect the therapeutic index. This article describes the relationship between potency and therapeutic index, and concludes that higher potency can potentially improve the therapeutic index. Therefore, both efficacy and safety should be considered when classifying inhaled corticosteroid regimens in terms of dose equivalence. The historical approach to dose equivalence in asthma treatment guidelines is not appropriate for the wider range of molecules, potencies and device/formulations now available. A more robust method is needed that incorporates pharmacological principles.
Keywords: Corticosteroid; dose equivalence; inhaled; potency; therapeutic index.
© 2015 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures

Similar articles
-
Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids.Immunol Allergy Clin North Am. 2005 Aug;25(3):469-88. doi: 10.1016/j.iac.2005.05.004. Immunol Allergy Clin North Am. 2005. PMID: 16054538 Review.
-
[Difficulties in determining of the equivalent doses of inhaled glucocorticoids].Pol Merkur Lekarski. 2010 Feb;28(164):134-7. Pol Merkur Lekarski. 2010. PMID: 20369743 Review. Polish.
-
Pharmaceutical characteristics that influence the clinical efficacy of inhaled corticosteroids.Ann Allergy Asthma Immunol. 2003 Oct;91(4):326-34; quiz 334-5, 404. doi: 10.1016/S1081-1206(10)61677-8. Ann Allergy Asthma Immunol. 2003. PMID: 14582810 Review.
-
Risk-benefit value of inhaled glucocorticoids: a pharmacokinetic/pharmacodynamic perspective.J Clin Pharmacol. 2004 Jan;44(1):37-47. doi: 10.1177/0091270003260334. J Clin Pharmacol. 2004. PMID: 14681340 Review.
-
Pharmacology and pharmacokinetics of topical corticosteroid derivatives used for asthma therapy.Am Rev Respir Dis. 1990 Feb;141(2 Pt 2):S44-51. Am Rev Respir Dis. 1990. PMID: 2178513 Review.
Cited by
-
Inhalable tobramycin EEG powder formulation for treating Pseudomonas aeruginosa-induced lung infection.Int J Pharm. 2024 Sep 5;662:124504. doi: 10.1016/j.ijpharm.2024.124504. Epub 2024 Jul 24. Int J Pharm. 2024. PMID: 39053676
-
Association between use of systemic and inhaled glucocorticoids and changes in brain volume and white matter microstructure: a cross-sectional study using data from the UK Biobank.BMJ Open. 2022 Aug 30;12(8):e062446. doi: 10.1136/bmjopen-2022-062446. BMJ Open. 2022. PMID: 36041764 Free PMC article.
-
Benefit:Risk Profile of Budesonide in Obstructive Airways Disease.Drugs. 2019 Nov;79(16):1757-1775. doi: 10.1007/s40265-019-01198-7. Drugs. 2019. PMID: 31549299 Free PMC article. Review.
-
Platelet-derived growth factor D expression in adrenal cells is modulated by corticosteroids: putative role in adrenal suppression.Pediatr Res. 2023 Jan;93(1):97-101. doi: 10.1038/s41390-022-02094-9. Epub 2022 May 14. Pediatr Res. 2023. PMID: 35568735 Free PMC article.
-
Fetal Concentrations of Budesonide and Fluticasone Propionate: a Study in Mice.AAPS J. 2019 Apr 16;21(4):53. doi: 10.1208/s12248-019-0313-2. AAPS J. 2019. PMID: 30993489
References
-
- Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–27. - PubMed
-
- Kelly HW. Establishing a therapeutic index for the inhaled corticosteroids: part I. Pharmacokinetic/pharmacodynamic comparison of the inhaled corticosteroids. J Allergy Clin Immunol. 1998;102:S36–51. - PubMed
-
- Kamada AK, Szefler SJ, Martin RJ, Boushey HA, Chinchilli VM, Drazen JM, Fish JE, Israel E, Lazarus SC, Lemanske RF. Issues in the use of inhaled glucocorticoids. The Asthma Clinical Research Network. Am J Respir Crit Care Med. 1996;153(6):1739–48. - PubMed
-
- Daley-Yates PT. The clinical utility of pharmacokinetics in demonstrating bioequivalence of locally acting orally inhaled drugs. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory Drug Delivery. Davis. River Grove: Healthcare International Publishing; 2010. pp. 273–83. 1.
-
- Hochhaus G, Mollmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic and pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37:881–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

