Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors
- PMID: 25805993
- PMCID: PMC4353375
- DOI: 10.3389/fphar.2015.00040
Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors
Abstract
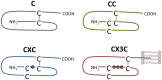
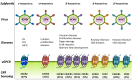
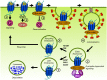
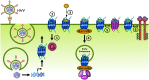
Human herpesviruses (HHVs) are widespread infectious pathogens that have been associated with proliferative and inflammatory diseases. During viral evolution, HHVs have pirated genes encoding viral G protein-coupled receptors (vGPCRs), which are expressed on infected host cells. These vGPCRs show highest homology to human chemokine receptors, which play a key role in the immune system. Importantly, vGPCRs have acquired unique properties such as constitutive activity and the ability to bind a broad range of human chemokines. This allows vGPCRs to hijack human proteins and modulate cellular signaling for the benefit of the virus, ultimately resulting in immune evasion and viral dissemination to establish a widespread and lifelong infection. Knowledge on the mechanisms by which herpesviruses reprogram cellular signaling might provide insight in the contribution of vGPCRs to viral survival and herpesvirus-associated pathologies.
Keywords: EBV; HCMV; KSHV; chemokine; chemokine receptor; human herpesvirus; review; viral GPCR.
Figures


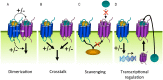
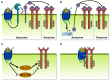
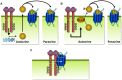
Similar articles
-
Herpesvirus-encoded GPCRs rewire cellular signaling.Mol Cell Endocrinol. 2011 Jan 15;331(2):179-84. doi: 10.1016/j.mce.2010.04.007. Epub 2010 Apr 14. Mol Cell Endocrinol. 2011. PMID: 20398729 Review.
-
Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses.Annu Rev Virol. 2022 Sep 29;9(1):329-351. doi: 10.1146/annurev-virology-100220-113942. Epub 2022 Jun 7. Annu Rev Virol. 2022. PMID: 35671566 Free PMC article. Review.
-
Herpesvirus-encoded G protein-coupled receptors as modulators of cellular function.Mol Pharmacol. 2009 Oct;76(4):692-701. doi: 10.1124/mol.109.057091. Epub 2009 Jul 1. Mol Pharmacol. 2009. PMID: 19570946 Review.
-
Constitutive activation of T cells by γ2-herpesviral GPCR through the interaction with cellular CXCR4.Biochim Biophys Acta Mol Cell Res. 2017 Jan;1864(1):1-11. doi: 10.1016/j.bbamcr.2016.10.008. Epub 2016 Oct 15. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27751885
-
HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks.Trends Pharmacol Sci. 2006 Jan;27(1):56-63. doi: 10.1016/j.tips.2005.11.006. Epub 2005 Dec 13. Trends Pharmacol Sci. 2006. PMID: 16352349 Review.
Cited by
-
Molecular Biology of KSHV in Relation to HIV/AIDS-Associated Oncogenesis.Cancer Treat Res. 2019;177:23-62. doi: 10.1007/978-3-030-03502-0_2. Cancer Treat Res. 2019. PMID: 30523620 Free PMC article.
-
Emerging roles of cytomegalovirus-encoded G protein-coupled receptors during lytic and latent infection.Med Microbiol Immunol. 2019 Aug;208(3-4):447-456. doi: 10.1007/s00430-019-00595-9. Epub 2019 Mar 21. Med Microbiol Immunol. 2019. PMID: 30900091 Review.
-
Human Cytomegalovirus US28 Is Important for Latent Infection of Hematopoietic Progenitor Cells.J Virol. 2015 Dec 30;90(6):2959-70. doi: 10.1128/JVI.02507-15. J Virol. 2015. PMID: 26719258 Free PMC article.
-
G protein coupled receptors signaling pathways implicate in inflammatory and immune response of rheumatoid arthritis.Inflamm Res. 2017 May;66(5):379-387. doi: 10.1007/s00011-016-1011-5. Epub 2016 Nov 23. Inflamm Res. 2017. PMID: 27878579 Review.
-
G-Protein coupled receptors: structure and function in drug discovery.RSC Adv. 2020 Oct 1;10(60):36337-36348. doi: 10.1039/d0ra08003a. eCollection 2020 Oct 1. RSC Adv. 2020. PMID: 35517958 Free PMC article. Review.
References
-
- Alderton F., Rakhit S., Kong K. C., Palmer T., Sambi B., Pyne S., et al. (2001). Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J. Biol. Chem. 276 28578–28585 10.1074/jbc.M102771200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

