The chromosomal passenger complex (CPC) as a key orchestrator of orderly mitotic exit and cytokinesis
- PMID: 25798441
- PMCID: PMC4350427
- DOI: 10.3389/fcell.2015.00014
The chromosomal passenger complex (CPC) as a key orchestrator of orderly mitotic exit and cytokinesis
Abstract
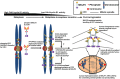
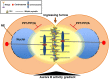
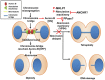
Understanding the molecular network of orderly mitotic exit to re-establish a functional interphase nucleus is critical because disordered mitotic exit inevitably leads to genomic instability. In contrast to the mechanisms of the entrance to mitosis, however, little is known about what controls the orderly exit from mitosis, particularly in mammalian cells. The chromosomal passenger complex (CPC), which is composed of Aurora B, INCENP, Borealin and Survivin, is one of the most widely studied and highly conserved hetero-tetrameric complexes. The CPC orchestrates proper chromosome segregation with cytokinesis by targeting to specific locations at different stages of mitosis. Recent studies reveal that controlling CPC localization and Aurora B kinase activity also serves as a key surveillance mechanism for the orderly mitotic exit. This ensures the reformation of a functional interphase nucleus from condensed mitotic chromosomes by delaying mitotic exit and cytokinetic processes in response to defects in chromosome segregation. In this review, we will summarize the latest insight into the molecular mechanisms that regulate CPC localization during mitotic exit and discuss how targeting Aurora B activity to different locations at different times impacts executing multiple mitotic exit events in order and recently proposed surveillance mechanisms. Finally, we briefly discuss the potential implication of deregulated Aurora B in inducing genomic damage and tumorigenesis with current efforts in targeting Aurora B activity for anti-cancer therapy.
Keywords: Aurora B kinase; abscission; chromosomal passenger complex; chromosome condensation; chromosome segregation; cytokinesis; mitotic exit; nuclear envelope reformation.
Figures

Similar articles
-
Chromosome segregation regulation in human zygotes: altered mitotic histone phosphorylation dynamics underlying centromeric targeting of the chromosomal passenger complex.Hum Reprod. 2015 Oct;30(10):2275-91. doi: 10.1093/humrep/dev186. Epub 2015 Jul 29. Hum Reprod. 2015. PMID: 26223676
-
APC/CCdh1 is required for the termination of chromosomal passenger complex activity upon mitotic exit.J Cell Sci. 2020 Sep 15;133(18):jcs251314. doi: 10.1242/jcs.251314. J Cell Sci. 2020. PMID: 32934012 Free PMC article.
-
Cell division control by the Chromosomal Passenger Complex.Exp Cell Res. 2012 Jul 15;318(12):1407-20. doi: 10.1016/j.yexcr.2012.03.015. Epub 2012 Mar 24. Exp Cell Res. 2012. PMID: 22472345 Review.
-
Aurora-C Interactions with Survivin and INCENP Reveal Shared and Distinct Features Compared with Aurora-B Chromosome Passenger Protein Complex.PLoS One. 2016 Jun 22;11(6):e0157305. doi: 10.1371/journal.pone.0157305. eCollection 2016. PLoS One. 2016. PMID: 27332895 Free PMC article.
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
Cited by
-
Coupling changes in cell shape to chromosome segregation.Nat Rev Mol Cell Biol. 2016 Aug;17(8):511-21. doi: 10.1038/nrm.2016.75. Epub 2016 Jun 29. Nat Rev Mol Cell Biol. 2016. PMID: 27353479 Review.
-
Functional Analysis of the Plant Chromosomal Passenger Complex.Plant Physiol. 2020 Aug;183(4):1586-1599. doi: 10.1104/pp.20.00344. Epub 2020 May 27. Plant Physiol. 2020. PMID: 32461300 Free PMC article.
-
Neuronal apoptosis inhibitory protein (NAIP) localizes to the cytokinetic machinery during cell division.Sci Rep. 2017 Jan 6;7:39981. doi: 10.1038/srep39981. Sci Rep. 2017. PMID: 28059125 Free PMC article.
-
Playing polo during mitosis: PLK1 takes the lead.Oncogene. 2017 Aug 24;36(34):4819-4827. doi: 10.1038/onc.2017.113. Epub 2017 Apr 24. Oncogene. 2017. PMID: 28436952 Review.
-
Specialize and Divide (Twice): Functions of Three Aurora Kinase Homologs in Mammalian Oocyte Meiotic Maturation.Trends Genet. 2017 May;33(5):349-363. doi: 10.1016/j.tig.2017.03.005. Epub 2017 Mar 27. Trends Genet. 2017. PMID: 28359584 Free PMC article. Review.
References
-
- Adams R. R., Maiato H., Earnshaw W. C., Carmena M. (2001). Essential roles of Drosophila inner centromere protein (INCENP) and Aurora-B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865–880. 10.1083/jcb.153.4.865 - DOI - PMC - PubMed
-
- Adams R. R., Wheatley S. P., Gouldsworthy A. M., Kandels-Lewis S. E., Carmena M., Smythe C., et al. . (2000). INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10, 1075–1078. 10.1016/S0960-9822(00)00673-4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

