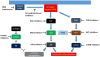
Treatment of NRAS-mutant melanoma
- PMID: 25796376
- PMCID: PMC4830486
- DOI: 10.1007/s11864-015-0330-z
Treatment of NRAS-mutant melanoma
Abstract
NRAS mutations in codons 12, 13, and 61 arise in 15-20 % of all melanomas. These alterations have been associated with aggressive clinical behavior and a poor prognosis. Until recently, there has been a paucity of promising genetically targeted therapy approaches for NRAS-mutant melanoma (and RAS-mutant malignancies in general). MEK inhibitors, particularly binimetinib, have shown activity in this cohort. Based on pre-clinical and early clinical studies, combining MEK inhibitors with agents inhibiting the cell cycling and the PI3K-AKT pathway appears to provide additional benefit. In particular, a strategy of MEK inhibition and CDK4/6 inhibition is likely to be a viable treatment option in the future, and is the most promising genetically targeted treatment strategy for NRAS-mutant melanoma developed to date. In addition, immune-based therapies have shown increasing activity in advanced melanoma and may be particularly effective in those with NRAS mutations. Combination strategies of immune and targeted therapies may also play a role in the future although clinical trials testing these approaches are in early stages.
Conflict of interest statement
Figures
Similar articles
-
NRAS-mutant melanoma: current challenges and future prospect.Onco Targets Ther. 2017 Aug 8;10:3941-3947. doi: 10.2147/OTT.S117121. eCollection 2017. Onco Targets Ther. 2017. PMID: 28860801 Free PMC article. Review.
-
Binimetinib for the treatment of NRAS-mutant melanoma.Expert Rev Anticancer Ther. 2017 Nov;17(11):985-990. doi: 10.1080/14737140.2017.1374177. Epub 2017 Sep 8. Expert Rev Anticancer Ther. 2017. PMID: 28851243 Review.
-
P53 and MITF/Bcl-2 identified as key pathways in the acquired resistance of NRAS-mutant melanoma to MEK inhibition.Eur J Cancer. 2017 Sep;83:154-165. doi: 10.1016/j.ejca.2017.06.033. Epub 2017 Jul 21. Eur J Cancer. 2017. PMID: 28738256
-
MEK inhibitors for the treatment of NRAS mutant melanoma.Drug Des Devel Ther. 2018 Aug 20;12:2553-2565. doi: 10.2147/DDDT.S131721. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30154648 Free PMC article. Review.
-
Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo.Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):4015-20. doi: 10.1073/pnas.1216013110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431193 Free PMC article.
Cited by
-
Knockdown of NEAT1 restrained the malignant progression of glioma stem cells by activating microRNA let-7e.Oncotarget. 2016 Sep 20;7(38):62208-62223. doi: 10.18632/oncotarget.11403. Oncotarget. 2016. PMID: 27556696 Free PMC article.
-
NRAS mutant melanoma: an overview for the clinician for melanoma management.Melanoma Manag. 2016 Mar;3(1):47-59. doi: 10.2217/mmt.15.40. Epub 2016 Feb 17. Melanoma Manag. 2016. PMID: 30190872 Free PMC article. Review.
-
High-throughput ex vivo drug testing identifies potential drugs and drug combinations for NRAS-positive malignant melanoma.Transl Oncol. 2022 Jan;15(1):101290. doi: 10.1016/j.tranon.2021.101290. Epub 2021 Nov 24. Transl Oncol. 2022. PMID: 34837846 Free PMC article.
-
Neural crest state activation in NRAS driven melanoma, but not in NRAS-driven melanocyte expansion.Dev Biol. 2019 May 15;449(2):107-114. doi: 10.1016/j.ydbio.2018.05.026. Epub 2018 Jun 5. Dev Biol. 2019. PMID: 29883661 Free PMC article.
-
NRAS-mutant melanoma: current challenges and future prospect.Onco Targets Ther. 2017 Aug 8;10:3941-3947. doi: 10.2147/OTT.S117121. eCollection 2017. Onco Targets Ther. 2017. PMID: 28860801 Free PMC article. Review.
References
-
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65. - PubMed
-
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249–56. This is the first clinical trial to show consistent activity of any genetically targeted therapy in NRAS mutant melanoma. - PubMed
-
- Sosman JA, Kittaneh M, Lolkema MPJ, Postow MA, Schwartz G, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity. J Clin Oncol. 2014;32:9009. Abstract.
-
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous