Oxidative and Nitrosative Stress and Histone Deacetylase-2 Activity in Exacerbations of COPD
- PMID: 25790167
- PMCID: PMC4700054
- DOI: 10.1378/chest.14-2637
Oxidative and Nitrosative Stress and Histone Deacetylase-2 Activity in Exacerbations of COPD
Abstract
Background: Respiratory virus infections are commonly associated with COPD exacerbations, but little is known about the mechanisms linking virus infection to exacerbations. Pathogenic mechanisms in stable COPD include oxidative and nitrosative stress and reduced activity of histone deacetylase-2 (HDAC2), but their roles in COPD exacerbations is unknown. We investigated oxidative and nitrosative stress (O&NS) and HDAC2 in COPD exacerbations using experimental rhinovirus infection.
Methods: Nine subjects with COPD (Global Initiative for Chronic Obstructive Lung Disease stage II), 10 smokers, and 11 nonsmokers were successfully infected with rhinovirus. Markers of O&NS-associated cellular damage, and inflammatory mediators and proteases were measured in sputum, and HDAC2 activity was measured in sputum and bronchoalveolar macrophages. In an in vitro model, monocyte-derived THP-1 cells were infected with rhinovirus and nitrosylation and activity of HDAC2 was measured.
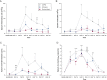
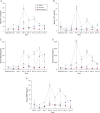
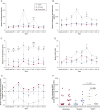
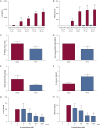
Results: Rhinovirus infection induced significant increases in airways inflammation and markers of O&NS in subjects with COPD. O&NS markers correlated with virus load and inflammatory markers. Macrophage HDAC2 activity was reduced during exacerbation and correlated inversely with virus load, inflammatory markers, and nitrosative stress. Sputum macrophage HDAC2 activity pre-infection was inversely associated with sputum virus load and inflammatory markers during exacerbation. Rhinovirus infection of monocytes induced nitrosylation of HDAC2 and reduced HDAC2 activity; inhibition of O&NS inhibited rhinovirus-induced inflammatory cytokines.
Conclusions: O&NS, airways inflammation, and impaired HDAC2 may be important mechanisms of virus-induced COPD exacerbations. Therapies targeting these mechanisms offer potential new treatments for COPD exacerbations.
Keywords: COPD; host defense; infection; viral disease.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation.Am J Respir Crit Care Med. 2011 Mar 15;183(6):734-42. doi: 10.1164/rccm.201006-0833OC. Epub 2010 Oct 1. Am J Respir Crit Care Med. 2011. PMID: 20889904 Free PMC article.
-
Bronchial mucosal inflammation and illness severity in response to experimental rhinovirus infection in COPD.J Allergy Clin Immunol. 2020 Oct;146(4):840-850.e7. doi: 10.1016/j.jaci.2020.03.021. Epub 2020 Apr 10. J Allergy Clin Immunol. 2020. PMID: 32283204 Free PMC article.
-
Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease.Am J Respir Crit Care Med. 2012 Dec 1;186(11):1117-24. doi: 10.1164/rccm.201205-0806OC. Epub 2012 Sep 28. Am J Respir Crit Care Med. 2012. PMID: 23024024 Free PMC article.
-
Human rhinovirus infection and COPD: role in exacerbations and potential for therapeutic targets.Expert Rev Respir Med. 2020 Aug;14(8):777-789. doi: 10.1080/17476348.2020.1764354. Epub 2020 Jun 4. Expert Rev Respir Med. 2020. PMID: 32498634 Review.
-
Experimental rhinovirus infection in COPD: implications for antiviral therapies.Antiviral Res. 2014 Feb;102:95-105. doi: 10.1016/j.antiviral.2013.12.006. Epub 2013 Dec 24. Antiviral Res. 2014. PMID: 24370732 Free PMC article. Review.
Cited by
-
Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease.Ann Med. 2021 Dec;53(1):135-150. doi: 10.1080/07853890.2020.1831050. Ann Med. 2021. PMID: 32997525 Free PMC article. Review.
-
Inflammation and infections in unreported chronic obstructive pulmonary disease exacerbations.Int J Chron Obstruct Pulmon Dis. 2019 Apr 10;14:823-832. doi: 10.2147/COPD.S191946. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31114182 Free PMC article.
-
The Biological Processes of Ferroptosis Involved in Pathogenesis of COVID-19 and Core Ferroptoic Genes Related With the Occurrence and Severity of This Disease.Evol Bioinform Online. 2023 Feb 13;19:11769343231153293. doi: 10.1177/11769343231153293. eCollection 2023. Evol Bioinform Online. 2023. PMID: 36820229 Free PMC article.
-
Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations.Nat Commun. 2018 Jun 8;9(1):2229. doi: 10.1038/s41467-018-04574-1. Nat Commun. 2018. PMID: 29884817 Free PMC article.
-
The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma.Ther Adv Respir Dis. 2016 Apr;10(2):158-74. doi: 10.1177/1753465815618113. Epub 2015 Nov 26. Ther Adv Respir Dis. 2016. PMID: 26611907 Free PMC article. Review.
References
-
- Vestbo J., Hurd S.S., Agustí A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. - PubMed
-
- Papi A., Bellettato C.M., Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. - PubMed
-
- Walters J.A., Gibson P.G., Wood-Baker R., Hannay M., Walters E.H. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;(1):CD001288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

