SUMO-mediated regulation of DNA damage repair and responses
- PMID: 25778614
- PMCID: PMC4380773
- DOI: 10.1016/j.tibs.2015.02.006
SUMO-mediated regulation of DNA damage repair and responses
Erratum in
- Trends Biochem Sci. 2015 Jun;40(6):338
Abstract
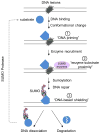
Sumoylation has important roles during DNA damage repair and responses. Recent broad-scope and substrate-based studies have shed light on the regulation and significance of sumoylation during these processes. An emerging paradigm is that sumoylation of many DNA metabolism proteins is controlled by DNA engagement. Such 'on-site modification' can explain low substrate modification levels and has important implications in sumoylation mechanisms and effects. New studies also suggest that sumoylation can regulate a process through an ensemble effect or via major substrates. Additionally, we describe new trends in the functional effects of sumoylation, such as bi-directional changes in biomolecule binding and multilevel coordination with other modifications. These emerging themes and models will stimulate our thinking and research in sumoylation and genome maintenance.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
SUMO-Mediated Regulation of Nuclear Functions and Signaling Processes.Mol Cell. 2018 Aug 2;71(3):409-418. doi: 10.1016/j.molcel.2018.07.027. Mol Cell. 2018. PMID: 30075142 Free PMC article. Review.
-
SUMOylation of damaged DNA-binding protein DDB2.Biochem Biophys Res Commun. 2013 Aug 16;438(1):26-31. doi: 10.1016/j.bbrc.2013.07.013. Epub 2013 Jul 13. Biochem Biophys Res Commun. 2013. PMID: 23860269
-
Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair.Cell. 2012 Nov 9;151(4):807-820. doi: 10.1016/j.cell.2012.10.021. Epub 2012 Nov 1. Cell. 2012. PMID: 23122649
-
Fifty shades of SUMO: its role in immunity and at the fulcrum of the growth-defence balance.Mol Plant Pathol. 2018 Jun;19(6):1537-1544. doi: 10.1111/mpp.12625. Epub 2018 Feb 13. Mol Plant Pathol. 2018. PMID: 29024335 Free PMC article. Review.
-
RanBP2-Mediated SUMOylation Promotes Human DNA Polymerase Lambda Nuclear Localization and DNA Repair.J Mol Biol. 2020 Jun 12;432(13):3965-3979. doi: 10.1016/j.jmb.2020.03.020. Epub 2020 Mar 26. J Mol Biol. 2020. PMID: 32224012
Cited by
-
The deubiquitinase USP36 promotes snoRNP group SUMOylation and is essential for ribosome biogenesis.EMBO Rep. 2021 Jun 4;22(6):e50684. doi: 10.15252/embr.202050684. Epub 2021 Apr 14. EMBO Rep. 2021. PMID: 33852194 Free PMC article.
-
PolySUMOylation by Siz2 and Mms21 triggers relocation of DNA breaks to nuclear pores through the Slx5/Slx8 STUbL.Genes Dev. 2016 Apr 15;30(8):931-45. doi: 10.1101/gad.277665.116. Epub 2016 Apr 7. Genes Dev. 2016. PMID: 27056668 Free PMC article.
-
Multifaceted roles of SUMO in DNA metabolism.Nucleus. 2024 Dec;15(1):2398450. doi: 10.1080/19491034.2024.2398450. Epub 2024 Sep 17. Nucleus. 2024. PMID: 39287196 Free PMC article. Review.
-
SUMO Interacting Motifs: Structure and Function.Cells. 2021 Oct 21;10(11):2825. doi: 10.3390/cells10112825. Cells. 2021. PMID: 34831049 Free PMC article. Review.
-
The post-translational modification, SUMOylation, and cancer (Review).Int J Oncol. 2018 Apr;52(4):1081-1094. doi: 10.3892/ijo.2018.4280. Epub 2018 Feb 22. Int J Oncol. 2018. PMID: 29484374 Free PMC article. Review.
References
-
- Kerscher O, et al. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. - PubMed
-
- Geiss-Friedlander R, et al. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. - PubMed
-
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. - PubMed
-
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

