Human Ebola virus infection results in substantial immune activation
- PMID: 25775592
- PMCID: PMC4403189
- DOI: 10.1073/pnas.1502619112
Human Ebola virus infection results in substantial immune activation
Abstract
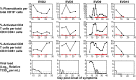
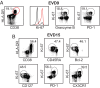
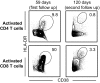
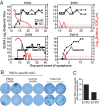
Four Ebola patients received care at Emory University Hospital, presenting a unique opportunity to examine the cellular immune responses during acute Ebola virus infection. We found striking activation of both B and T cells in all four patients. Plasmablast frequencies were 10-50% of B cells, compared with less than 1% in healthy individuals. Many of these proliferating plasmablasts were IgG-positive, and this finding coincided with the presence of Ebola virus-specific IgG in the serum. Activated CD4 T cells ranged from 5 to 30%, compared with 1-2% in healthy controls. The most pronounced responses were seen in CD8 T cells, with over 50% of the CD8 T cells expressing markers of activation and proliferation. Taken together, these results suggest that all four patients developed robust immune responses during the acute phase of Ebola virus infection, a finding that would not have been predicted based on our current assumptions about the highly immunosuppressive nature of Ebola virus. Also, quite surprisingly, we found sustained immune activation after the virus was cleared from the plasma, observed most strikingly in the persistence of activated CD8 T cells, even 1 mo after the patients' discharge from the hospital. These results suggest continued antigen stimulation after resolution of the disease. From these convalescent time points, we identified CD4 and CD8 T-cell responses to several Ebola virus proteins, most notably the viral nucleoprotein. Knowledge of the viral proteins targeted by T cells during natural infection should be useful in designing vaccines against Ebola virus.
Keywords: Ebola infection; T cells; human immune response; immune activation; plasmablasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Robust and sustained immune activation in human Ebola virus infection.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4518-9. doi: 10.1073/pnas.1503864112. Epub 2015 Apr 7. Proc Natl Acad Sci U S A. 2015. PMID: 25852148 Free PMC article. No abstract available.
Similar articles
-
Persistent infection with ebola virus under conditions of partial immunity.J Virol. 2004 Jan;78(2):958-67. doi: 10.1128/jvi.78.2.958-967.2004. J Virol. 2004. PMID: 14694127 Free PMC article.
-
Robust and sustained immune activation in human Ebola virus infection.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4518-9. doi: 10.1073/pnas.1503864112. Epub 2015 Apr 7. Proc Natl Acad Sci U S A. 2015. PMID: 25852148 Free PMC article. No abstract available.
-
Functional CD8+ T cell responses in lethal Ebola virus infection.J Immunol. 2008 Mar 15;180(6):4058-66. doi: 10.4049/jimmunol.180.6.4058. J Immunol. 2008. PMID: 18322215
-
Camouflage and misdirection: the full-on assault of ebola virus disease.Cell. 2014 Oct 23;159(3):477-86. doi: 10.1016/j.cell.2014.10.006. Epub 2014 Oct 16. Cell. 2014. PMID: 25417101 Free PMC article. Review.
-
Immunopathology of highly virulent pathogens: insights from Ebola virus.Nat Immunol. 2007 Nov;8(11):1159-64. doi: 10.1038/ni1519. Nat Immunol. 2007. PMID: 17952040 Free PMC article. Review.
Cited by
-
Immune phenotypes that are associated with subsequent COVID-19 severity inferred from post-recovery samples.Nat Commun. 2022 Nov 25;13(1):7255. doi: 10.1038/s41467-022-34638-2. Nat Commun. 2022. PMID: 36433939 Free PMC article.
-
Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients.Nat Commun. 2021 May 11;12(1):2691. doi: 10.1038/s41467-021-23018-x. Nat Commun. 2021. PMID: 33976217 Free PMC article.
-
Immune responses during COVID-19 infection.Oncoimmunology. 2020 Aug 25;9(1):1807836. doi: 10.1080/2162402X.2020.1807836. Oncoimmunology. 2020. PMID: 32939324 Free PMC article. Review.
-
Severe Human Lassa Fever Is Characterized by Nonspecific T-Cell Activation and Lymphocyte Homing to Inflamed Tissues.J Virol. 2020 Oct 14;94(21):e01367-20. doi: 10.1128/JVI.01367-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817220 Free PMC article.
-
Kinetics of Soluble Mediators of the Host Response in Ebola Virus Disease.J Infect Dis. 2018 Nov 22;218(suppl_5):S496-S503. doi: 10.1093/infdis/jiy429. J Infect Dis. 2018. PMID: 30101349 Free PMC article.
References
-
- Sanchez A, Geisbert T, Feldmann H. 2007. Filoviridae: Marburg and Ebola Viruses (Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia), 5th Ed.
-
- Baize S, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5(4):423–426. - PubMed
-
- Villinger F, et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–S191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

