Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy
- PMID: 25775028
- PMCID: PMC4353464
- DOI: 10.1186/s40478-015-0186-2
Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy
Abstract
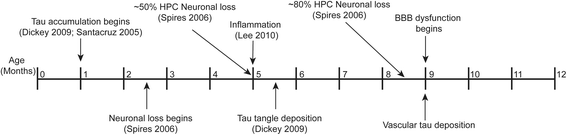
Introduction: The blood-brain barrier (BBB) is damaged in tauopathies, including progressive supranuclear palsy (PSP) and Alzheimer's disease (AD), which is thought to contribute to pathogenesis later in the disease course. In AD, BBB dysfunction has been associated with amyloid beta (Aß) pathology, but the role of tau in this process is not well characterized. Since increased BBB permeability is found in tauopathies without Aß pathology, like PSP, we suspected that tau accumulation alone could not only be sufficient, but even more important than Aß for BBB damage.
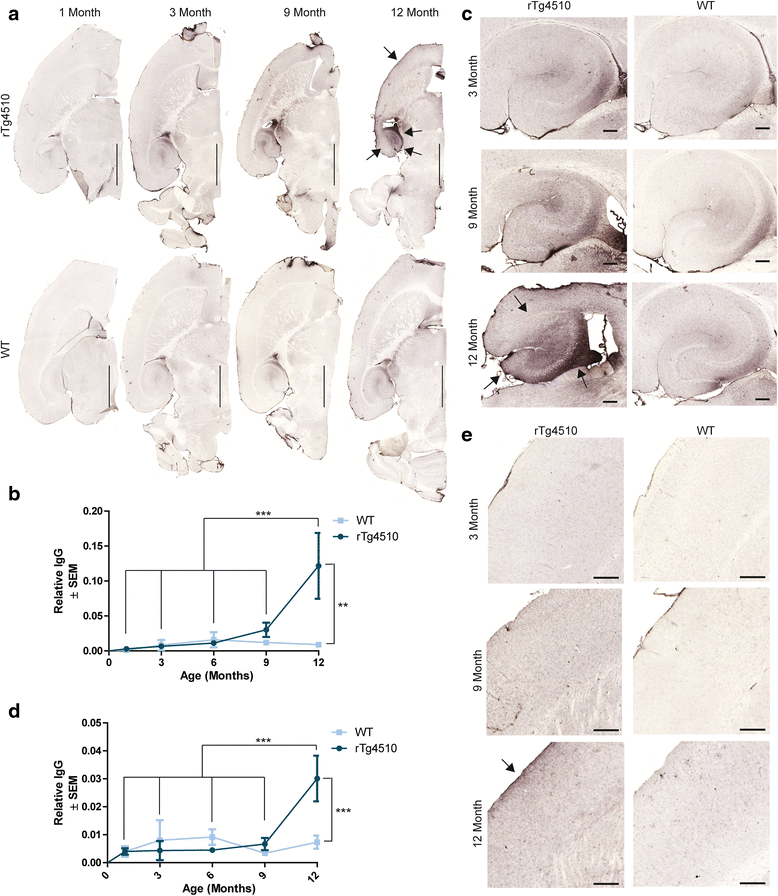
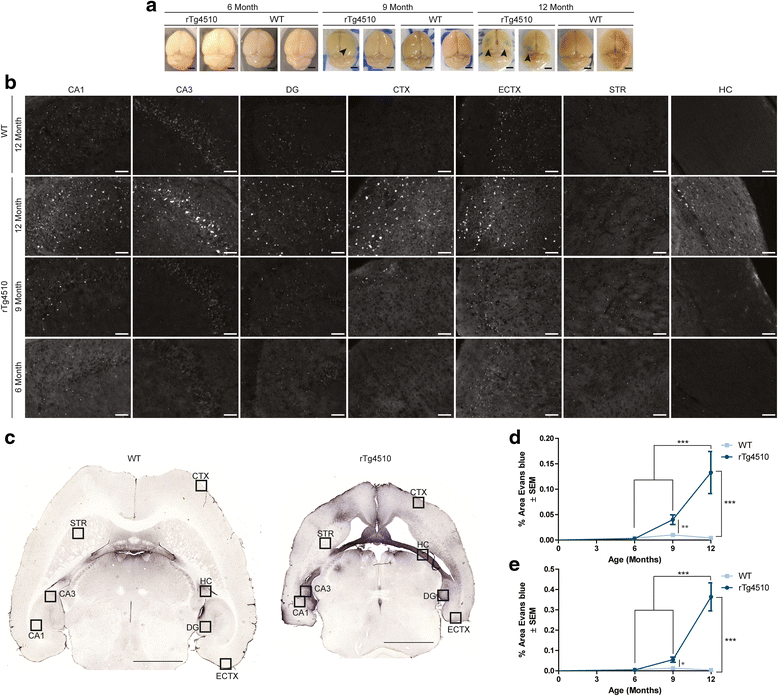
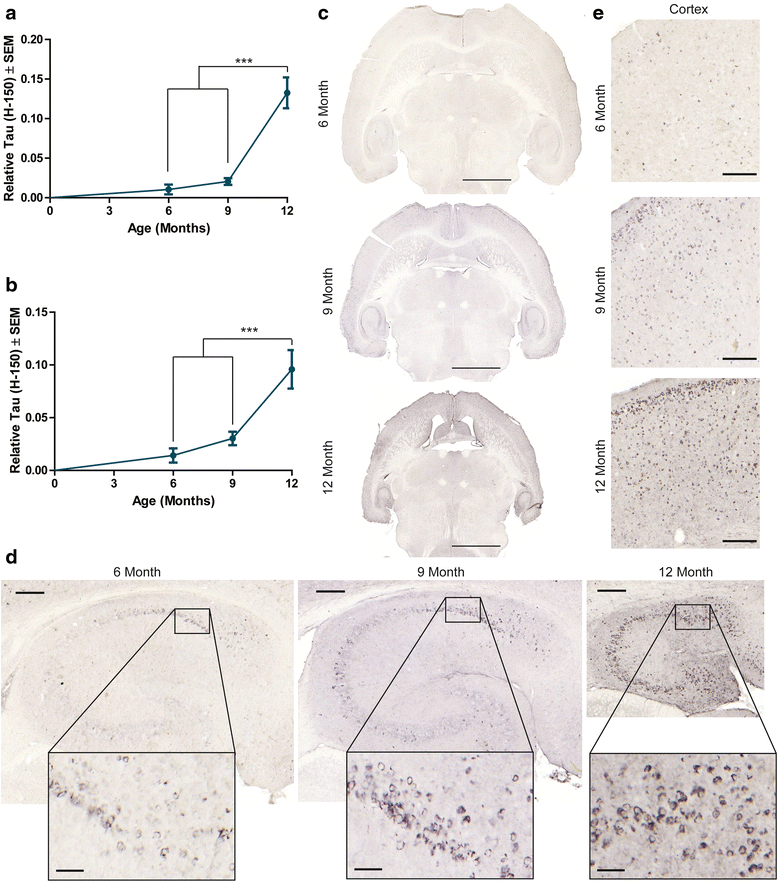
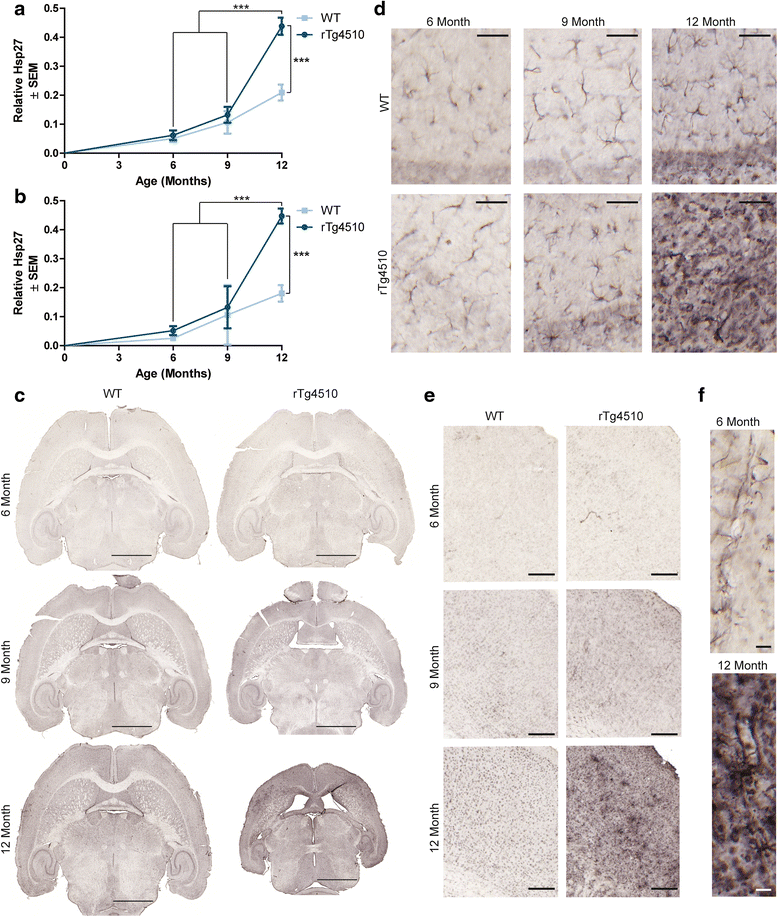
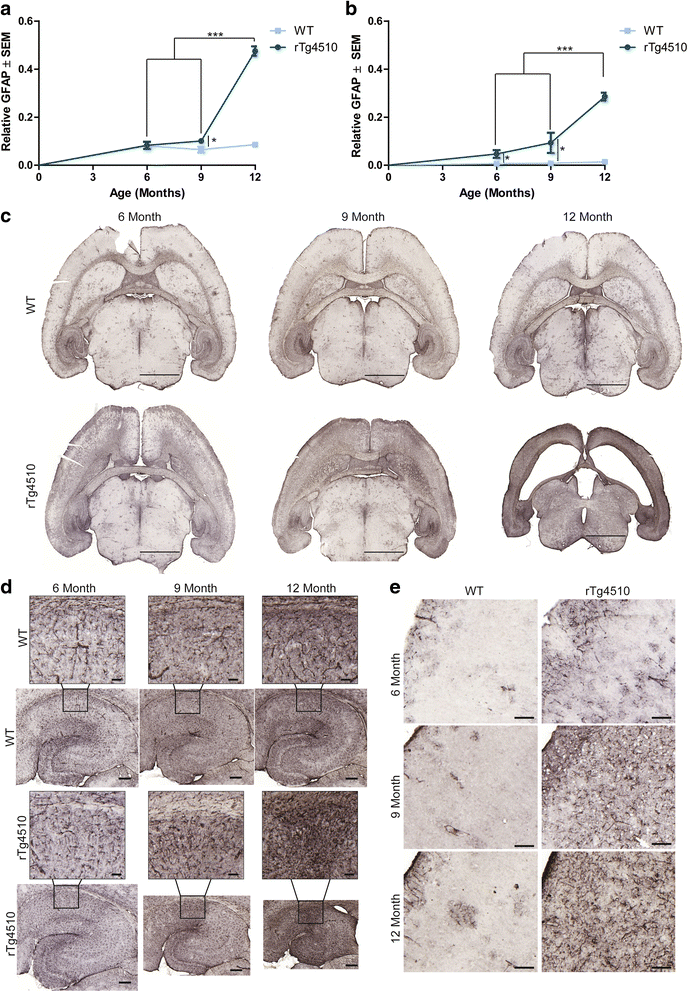
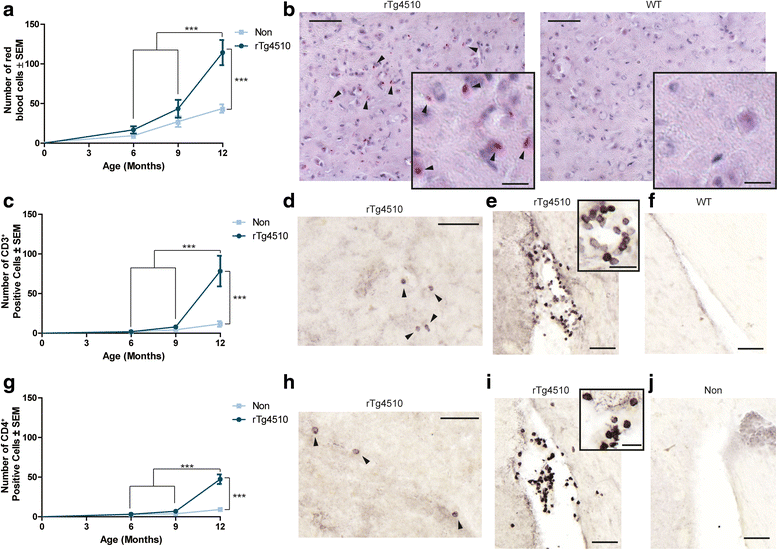
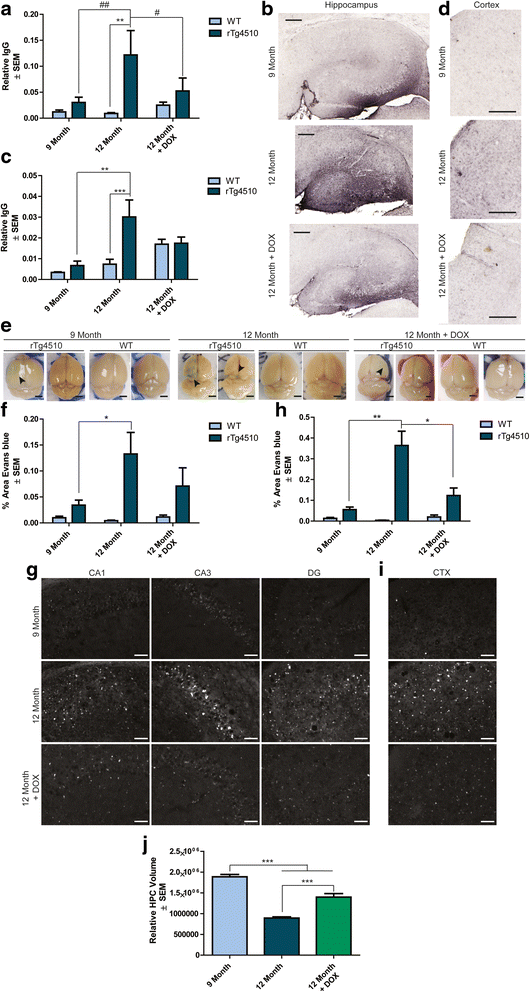
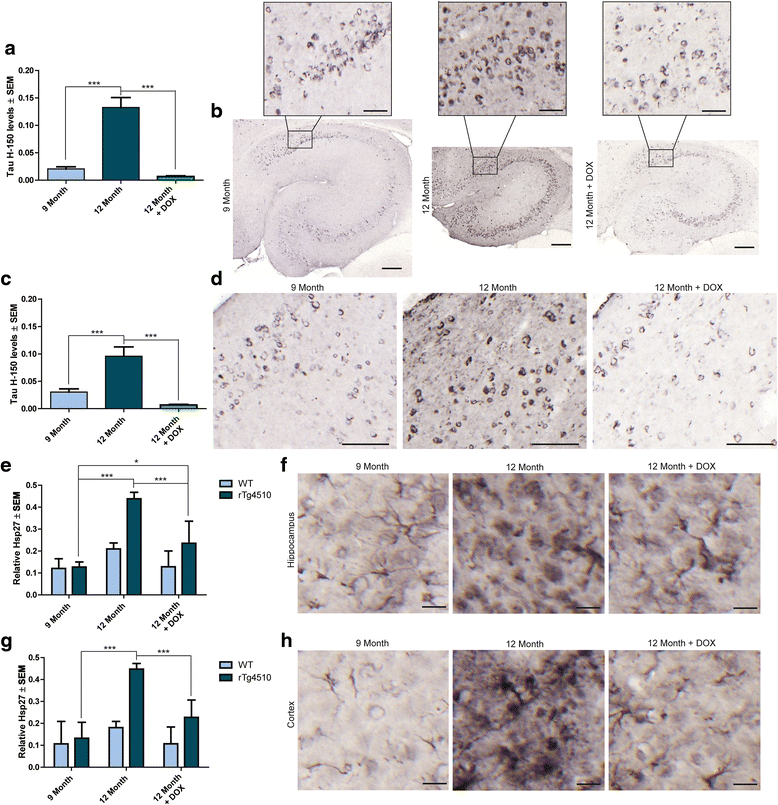
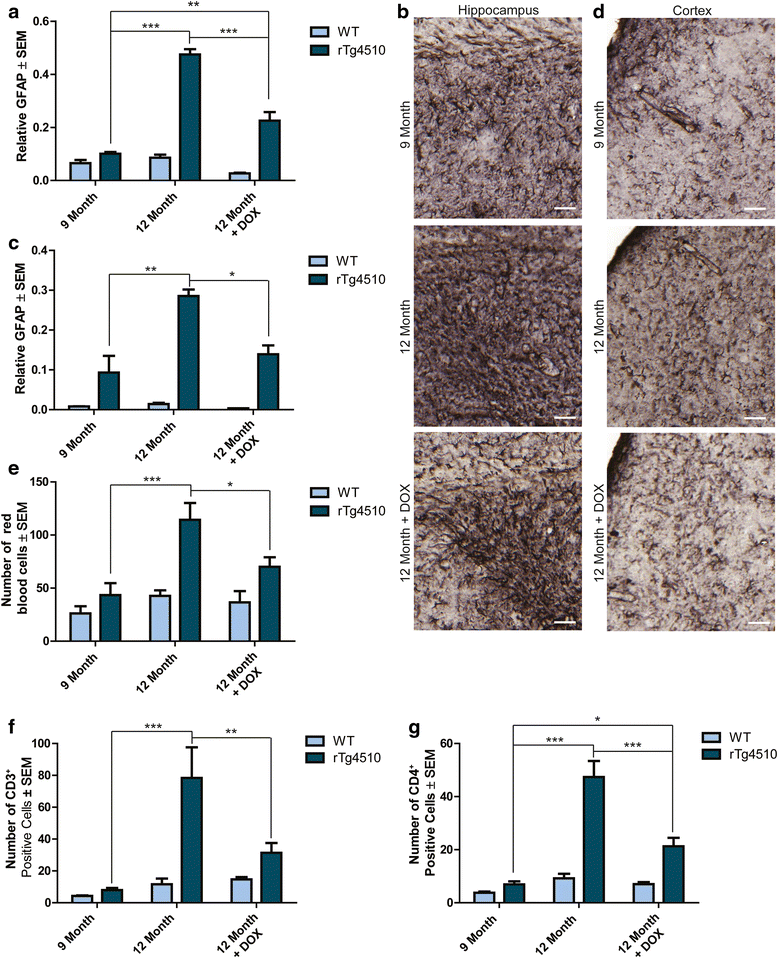
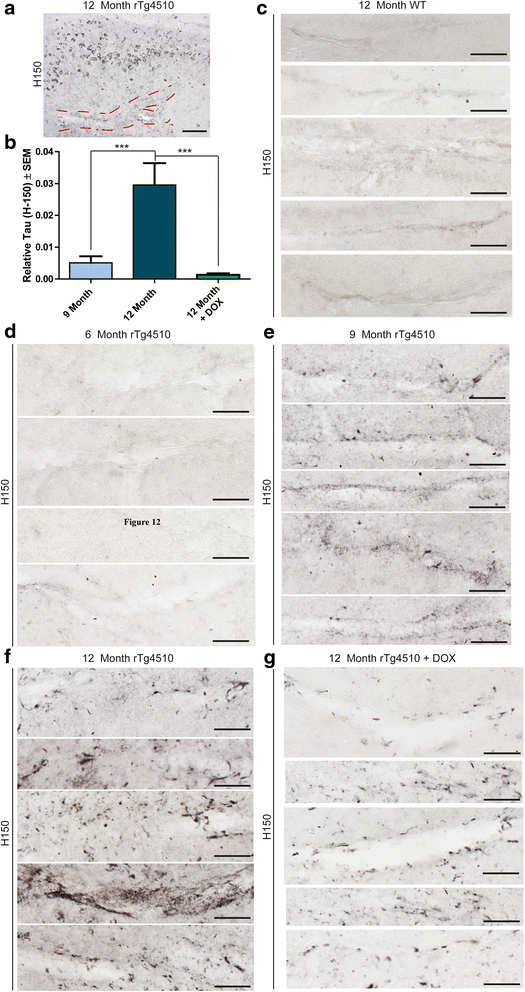
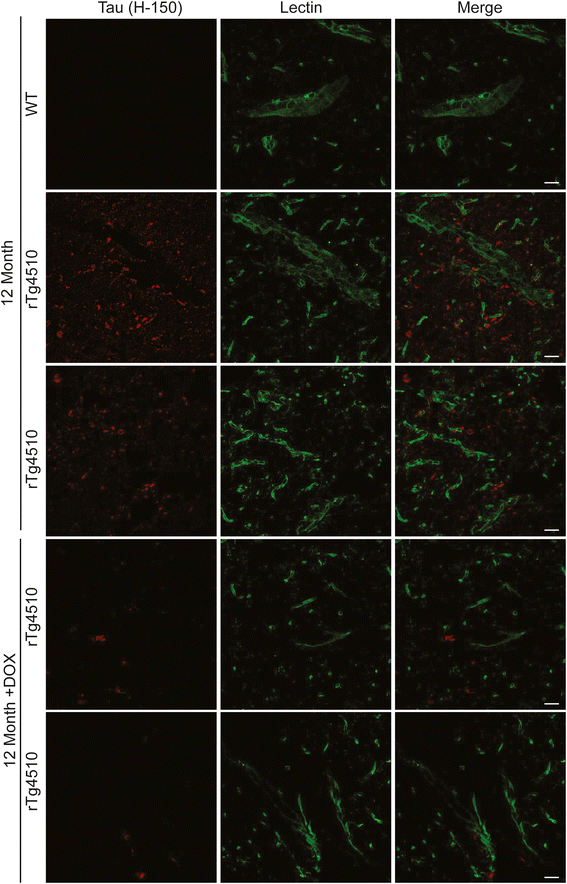
Results: Longitudinal evaluation of brain tissue from the tetracycline-regulatable rTg4510 tau transgenic mouse model showed progressive IgG, T cell and red blood cell infiltration. The Evans blue (EB) dye that is excluded from the brain when the BBB is intact also permeated the brains of rTg4510 mice following peripheral administration, indicative of a bonafide BBB defect, but this was only evident later in life. Thus, despite the marked brain atrophy and inflammation that occurs earlier in this model, BBB integrity is maintained. Interestingly, BBB dysfunction emerged at the same time that perivascular tau emerged around major hippocampal blood vessels. However, when tau expression was suppressed using doxycycline, BBB integrity was preserved, suggesting that the BBB can be stabilized in a tauopathic brain by reducing tau levels.
Conclusions: For the first time, these data demonstrate that tau alone can initiate breakdown of the BBB, but the BBB is remarkably resilient, maintaining its integrity in the face of marked brain atrophy, neuroinflammation and toxic tau accumulation. Moreover, the BBB can recover integrity when tau levels are reduced. Thus, late stage interventions targeting tau may slow the vascular contributions to cognitive impairment and dementia that occur in tauopathies.
Figures
Similar articles
-
Early intervention of tau pathology prevents behavioral changes in the rTg4510 mouse model of tauopathy.PLoS One. 2018 Apr 6;13(4):e0195486. doi: 10.1371/journal.pone.0195486. eCollection 2018. PLoS One. 2018. PMID: 29624602 Free PMC article.
-
Tracking progressive pathological and functional decline in the rTg4510 mouse model of tauopathy.Alzheimers Res Ther. 2017 Sep 20;9(1):77. doi: 10.1186/s13195-017-0306-2. Alzheimers Res Ther. 2017. PMID: 28931441 Free PMC article.
-
A novel transgenic mouse expressing double mutant tau driven by its natural promoter exhibits tauopathy characteristics.Exp Neurol. 2008 Jul;212(1):71-84. doi: 10.1016/j.expneurol.2008.03.007. Epub 2008 Mar 21. Exp Neurol. 2008. PMID: 18490011
-
Tauopathies - Focus on Changes at the Neurovascular Unit.Curr Alzheimer Res. 2017;14(7):790-801. doi: 10.2174/1567205014666170203143336. Curr Alzheimer Res. 2017. PMID: 28164774 Review.
-
Tau Protein and Its Role in Blood-Brain Barrier Dysfunction.Front Mol Neurosci. 2020 Sep 30;13:570045. doi: 10.3389/fnmol.2020.570045. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33100967 Free PMC article. Review.
Cited by
-
Tau at the interface between neurodegeneration and neuroinflammation.Genes Immun. 2020 Nov;21(5):288-300. doi: 10.1038/s41435-020-00113-5. Epub 2020 Oct 3. Genes Immun. 2020. PMID: 33011744 Review.
-
The Delivery Challenge in Neurodegenerative Disorders: The Nanoparticles Role in Alzheimer's Disease Therapeutics and Diagnostics.Pharmaceutics. 2018 Oct 17;10(4):190. doi: 10.3390/pharmaceutics10040190. Pharmaceutics. 2018. PMID: 30336640 Free PMC article. Review.
-
In vivo imaging and analysis of cerebrovascular hemodynamic responses and tissue oxygenation in the mouse brain.Nat Protoc. 2018 Jun;13(6):1377-1402. doi: 10.1038/nprot.2018.034. Epub 2018 May 24. Nat Protoc. 2018. PMID: 29844521 Free PMC article.
-
The Alzheimer's disease risk factor CD2AP maintains blood-brain barrier integrity.Hum Mol Genet. 2015 Dec 1;24(23):6667-74. doi: 10.1093/hmg/ddv371. Epub 2015 Sep 10. Hum Mol Genet. 2015. PMID: 26358779 Free PMC article.
-
Endothelin A receptors contribute to senescence of brain microvascular endothelial cells.Can J Physiol Pharmacol. 2022 Dec 1;100(12):1087-1096. doi: 10.1139/cjpp-2022-0071. Epub 2022 Nov 17. Can J Physiol Pharmacol. 2022. PMID: 36384316 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

