A novel therapy for melanoma developed in mice: transformation of melanoma into dendritic cells with Listeria monocytogenes
- PMID: 25760947
- PMCID: PMC4356589
- DOI: 10.1371/journal.pone.0117923
A novel therapy for melanoma developed in mice: transformation of melanoma into dendritic cells with Listeria monocytogenes
Abstract
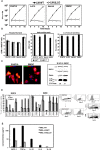
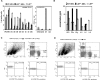
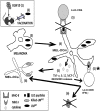
Listeria monocytogenes is a gram-positive bacteria and human pathogen widely used in cancer immunotherapy because of its capacity to induce a specific cytotoxic T cell response in tumours. This bacterial pathogen strongly induces innate and specific immunity with the potential to overcome tumour induced tolerance and weak immunogenicity. Here, we propose a Listeria based vaccination for melanoma based in its tropism for these tumour cells and its ability to transform in vitro and in vivo melanoma cells into matured and activated dendritic cells with competent microbicidal and antigen processing abilities. This Listeria based vaccination using low doses of the pathogen caused melanoma regression by apoptosis as well as bacterial clearance. Vaccination efficacy is LLO dependent and implies the reduction of LLO-specific CD4+ T cell responses, strong stimulation of innate pro-inflammatory immune cells and a prevalence of LLO-specific CD8+ T cells involved in tumour regression and Listeria elimination. These results support the use of low doses of pathogenic Listeria as safe melanoma therapeutic vaccines that do not require antibiotics for bacterial removal.
Conflict of interest statement
Figures

Similar articles
-
Central nervous system tumor immunity generated by a recombinant listeria monocytogenes vaccine targeting tyrosinase related protein-2 and real-time imaging of intracranial tumor burden.Neurosurgery. 2006 Jan;58(1):169-78; discussion 169-78. doi: 10.1227/01.neu.0000192367.29047.64. Neurosurgery. 2006. PMID: 16385341
-
A gold glyco-nanoparticle carrying a Listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection.Vaccine. 2015 Mar 17;33(12):1465-73. doi: 10.1016/j.vaccine.2015.01.062. Epub 2015 Feb 7. Vaccine. 2015. PMID: 25659269
-
Listeria monocytogenes activated dendritic cell based vaccine for prevention of experimental tumor in mice.Iran J Immunol. 2008 Mar;5(1):36-44. Iran J Immunol. 2008. PMID: 18319523
-
Review: dendritic cell immunotherapy for melanoma.Cancer Biother Radiopharm. 1999 Feb;14(1):11-22. doi: 10.1089/cbr.1999.14.11. Cancer Biother Radiopharm. 1999. PMID: 10850282 Review.
-
Vaccines and melanoma.Hematol Oncol Clin North Am. 2014 Jun;28(3):559-69. doi: 10.1016/j.hoc.2014.02.008. Epub 2014 Apr 3. Hematol Oncol Clin North Am. 2014. PMID: 24880947 Review.
Cited by
-
Biomarker Tools to Design Clinical Vaccines Determined from a Study of Annual Listeriosis Incidence in Northern Spain.Front Immunol. 2016 Nov 29;7:541. doi: 10.3389/fimmu.2016.00541. eCollection 2016. Front Immunol. 2016. PMID: 27965668 Free PMC article.
-
Exceptional antineoplastic activity of a dendritic-cell-targeted vaccine loaded with a Listeria peptide proposed against metastatic melanoma.Oncotarget. 2016 Mar 29;7(13):16855-65. doi: 10.18632/oncotarget.7806. Oncotarget. 2016. PMID: 26942874 Free PMC article.
-
Attenuated Bacteria as Immunotherapeutic Tools for Cancer Treatment.Front Oncol. 2018 May 1;8:136. doi: 10.3389/fonc.2018.00136. eCollection 2018. Front Oncol. 2018. PMID: 29765907 Free PMC article.
-
GNP-GAPDH1-22 nanovaccines prevent neonatal listeriosis by blocking microglial apoptosis and bacterial dissemination.Oncotarget. 2017 Jul 20;8(33):53916-53934. doi: 10.18632/oncotarget.19405. eCollection 2017 Aug 15. Oncotarget. 2017. PMID: 28903312 Free PMC article.
-
Dendritic cell therapy in melanoma.Ann Transl Med. 2017 Oct;5(19):386. doi: 10.21037/atm.2017.06.13. Ann Transl Med. 2017. PMID: 29114544 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

