Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria
- PMID: 25759513
- PMCID: PMC4345178
- DOI: 10.3164/jcbn.14-134
Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria
Abstract
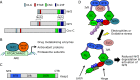
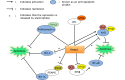
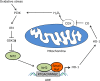
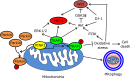
Nuclear factor erythroid-derived 2-related factor 2 (Nrf2) was originally identified as a positive regulator of drug detoxifying enzyme gene expression during exposure to environmental electrophiles. Currently, Nrf2 is known to regulate the expression of hundreds of cytoprotective genes to counteract endogenously or exogenously generated oxidative stress. Furthermore, when activated in human tumors by somatic mutations, Nrf2 confers growth advantages and chemoresistance by regulating genes involved in various processes such as the pentose phosphate pathway and nucleotide synthesis in addition to antioxidant proteins. Interestingly, increasing evidence shows that Nrf2 is associated with mitochondrial biogenesis during environmental stresses in certain tissues such as the heart. Furthermore, SKN-1, a functional homolog of Nrf2 in C. elegans, is activated by mitochondrial reactive oxygen species and extends life span by promoting mitochondrial homeostasis (i.e., mitohormesis). Similarly, Nrf2 activation was recently observed in the heart of surfeit locus protein 1 (Surf1) -/- mice in which cellular respiration was decreased due to cytochrome c oxidase defects. In this review, we critically examine the relationship between Nrf2 and mitochondria and argue that the Nrf2 stress pathway intimately communicates with mitochondria to maintain cellular homeostasis during oxidative stress.
Keywords: NRF-1; Nrf2; heme oxygenase-1; mitochondria; p62.
Figures
Similar articles
-
p62/SQSTM1 protects against cisplatin-induced oxidative stress in kidneys by mediating the cross talk between autophagy and the Keap1-Nrf2 signalling pathway.Free Radic Res. 2019 Jul;53(7):800-814. doi: 10.1080/10715762.2019.1635251. Epub 2019 Jul 8. Free Radic Res. 2019. PMID: 31223046
-
Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria.Toxicol Appl Pharmacol. 2018 Nov 15;359:24-33. doi: 10.1016/j.taap.2018.09.014. Epub 2018 Sep 18. Toxicol Appl Pharmacol. 2018. PMID: 30236989 Review.
-
Severe Fever with Thrombocytopenia Syndrome Virus NSs Interacts with TRIM21 To Activate the p62-Keap1-Nrf2 Pathway.J Virol. 2020 Feb 28;94(6):e01684-19. doi: 10.1128/JVI.01684-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852783 Free PMC article.
-
Emerging evidence for crosstalk between Nrf2 and mitochondria in physiological homeostasis and in heart disease.Arch Pharm Res. 2020 Mar;43(3):286-296. doi: 10.1007/s12272-019-01188-z. Epub 2019 Nov 11. Arch Pharm Res. 2020. PMID: 31712965 Review.
-
Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts.Cancers (Basel). 2020 Jul 7;12(7):1822. doi: 10.3390/cancers12071822. Cancers (Basel). 2020. PMID: 32645959 Free PMC article. Review.
Cited by
-
Association of Nrf2 with airway pathogenesis: lessons learned from genetic mouse models.Arch Toxicol. 2015 Nov;89(11):1931-57. doi: 10.1007/s00204-015-1557-y. Epub 2015 Jul 21. Arch Toxicol. 2015. PMID: 26194645 Free PMC article. Review.
-
Xuesaitong Combined with Dexmedetomidine Improves Cerebral Ischemia-Reperfusion Injury in Rats by Activating Keap1/Nrf2 Signaling and Mitophagy in Hippocampal Tissue.Oxid Med Cell Longev. 2022 Dec 7;2022:5126042. doi: 10.1155/2022/5126042. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36531207 Free PMC article.
-
Epigenetic versus Genetic Deregulation of the KEAP1/NRF2 Axis in Solid Tumors: Focus on Methylation and Noncoding RNAs.Oxid Med Cell Longev. 2018 Mar 13;2018:2492063. doi: 10.1155/2018/2492063. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29643973 Free PMC article. Review.
-
Angiotensin receptor blockade improves cardiac mitochondrial activity in response to an acute glucose load in obese insulin resistant rats.Redox Biol. 2018 Apr;14:371-378. doi: 10.1016/j.redox.2017.10.005. Epub 2017 Oct 7. Redox Biol. 2018. PMID: 29049981 Free PMC article.
-
The antioxidant icariin protects porcine oocytes from age-related damage in vitro.Anim Biosci. 2021 Apr;34(4):546-557. doi: 10.5713/ajas.20.0046. Epub 2020 May 12. Anim Biosci. 2021. PMID: 32777912 Free PMC article.
References
-
- Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol. 2011;41:1196–1202. - PubMed
-
- Indo HP, Davidson M, Yen HC, et al. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. - PubMed
-
- Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 2010;13:1665–1678. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

