Anti-IL-17 therapy restricts and reverses late-term corneal allorejection
- PMID: 25754737
- PMCID: PMC4390481
- DOI: 10.4049/jimmunol.1401922
Anti-IL-17 therapy restricts and reverses late-term corneal allorejection
Abstract
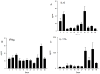
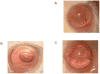
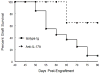
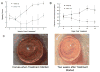
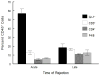
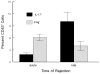
Corneal allograft rejection has been described as a Th1-mediated process involving IFN-γ production. However, recent evidence also implicated IL-17 as being involved in acute corneal allograft responses. Our data support that IL-17 is involved in early acute corneal allograft acceptance. However, we decided to extend these studies to include a later phase of rejection in which there is a peak of IL-17 production that is >15-fold higher than that seen during acute rejection and occurs >45 d postengraftment at the onset of late-term rejection. We demonstrate that neutralizing IL-17A at this time significantly reduced corneal graft rejection. Surprisingly, when corneal grafts that are undergoing this later phase of rejection are treated with anti-IL-17A, there is a reversal of both opacity and neovascularization. Compared with the early phase of rejection, the cellular infiltrate is significantly less, with a greatly reduced presence of Gr-1(+) neutrophils and a relative increase in CD4(+) T cells and macrophages. We went on to identify that the cells expressing IL-17 were CD4(+) IL-17(+) T cells and, somewhat surprisingly, IL-17(+) F4/80(+) macrophages within the rejecting corneal allografts. Taken together, these findings describe a distinct late phase of corneal allograft rejection that is likely mediated by Th17 cells; therapeutic neutralization of IL-17A reverses this rejection. This further suggests that IL-17 might serve as an excellent therapeutic target to reduce this form of corneal allograft rejection.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no conflict of interest with this work.
Figures





Similar articles
-
A pathogenic role of IL- 17 at the early stage of corneal allograft rejection.Transpl Immunol. 2009 Jul;21(3):155-61. doi: 10.1016/j.trim.2009.03.006. Epub 2009 Apr 7. Transpl Immunol. 2009. PMID: 19358887
-
MHC-matched corneal allograft rejection in an IFN-gamma/IL-17-independent manner in C57BL/6 mice.Invest Ophthalmol Vis Sci. 2009 May;50(5):2139-46. doi: 10.1167/iovs.08-2993. Epub 2009 Jan 10. Invest Ophthalmol Vis Sci. 2009. PMID: 19136699
-
Neutralization of mouse interleukin-17 bioactivity inhibits corneal allograft rejection.Mol Vis. 2011;17:2148-56. Epub 2011 Aug 11. Mol Vis. 2011. PMID: 21850190 Free PMC article.
-
Experimental corneal allograft rejection.Immunol Res. 2002;25(1):1-26. doi: 10.1385/IR:25:1:01. Immunol Res. 2002. PMID: 11868932 Review.
-
Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection.Cornea. 2000 Sep;19(5):625-43. doi: 10.1097/00003226-200009000-00008. Cornea. 2000. PMID: 11009315 Review.
Cited by
-
Therapeutic approaches for induction of tolerance and immune quiescence in corneal allotransplantation.Cell Mol Life Sci. 2018 May;75(9):1509-1520. doi: 10.1007/s00018-017-2739-y. Epub 2018 Jan 6. Cell Mol Life Sci. 2018. PMID: 29307015 Free PMC article. Review.
-
Human CD4 cytotoxic T lymphocytes mediate potent tumor control in humanized immune system mice.Commun Biol. 2023 Apr 25;6(1):447. doi: 10.1038/s42003-023-04812-3. Commun Biol. 2023. PMID: 37185301 Free PMC article.
-
Anti-interleukin-17A and anti-interleukin-23 antibodies may be effective against Alzheimer's disease: Role of neutrophils in the pathogenesis.Brain Behav. 2020 Jan;10(1):e01504. doi: 10.1002/brb3.1504. Epub 2019 Dec 17. Brain Behav. 2020. PMID: 31849180 Free PMC article. Review.
-
Tocilizumab promotes corneal allograft survival in rats by modulating Treg-Th17 balance.Int J Ophthalmol. 2019 Dec 18;12(12):1823-1831. doi: 10.18240/ijo.2019.12.02. eCollection 2019. Int J Ophthalmol. 2019. PMID: 31850163 Free PMC article.
-
Pathophysiology and classification of primary graft dysfunction after lung transplantation.J Thorac Dis. 2017 Oct;9(10):4084-4097. doi: 10.21037/jtd.2017.09.09. J Thorac Dis. 2017. PMID: 29268419 Free PMC article. Review.
References
-
- Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmol. 1998;105:1855–1865. - PubMed
-
- Yamagami S, Suzuki Y, Tsuru T. Risk factors for graft failure in penetrating keratoplasty. Acta Ophthalmol Scand. 1996;74:584–588. - PubMed
-
- Inoue K, Amano S, Oshika T, Tsuru T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scand. 2001;79:251–255. - PubMed
-
- Stuart PM, Yin XT, Plambeck S, Pan F, Ferguson TA. The role of Fas ligand as an effector molecule in corneal graft rejection. Eur J Immunol. 2005;35:2591–2597. - PubMed
-
- Gong HQ, Gao H, Xie LX, Shi WY. Ultrastructure changes in chronic corneal allograft dysfunction after penetrating keratoplasty. Zhonghua Yanke Zazhi. 2007;43:307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

