Alternative mechanisms for talin to mediate integrin function
- PMID: 25754646
- PMCID: PMC4386027
- DOI: 10.1016/j.cub.2015.01.043
Alternative mechanisms for talin to mediate integrin function
Abstract
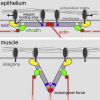
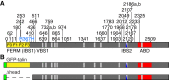
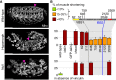
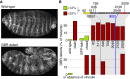
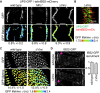
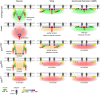
Cell-matrix adhesion is essential for building animals, promoting tissue cohesion, and enabling cells to migrate and resist mechanical force. Talin is an intracellular protein that is critical for linking integrin extracellular-matrix receptors to the actin cytoskeleton. A key question raised by structure-function studies is whether talin, which is critical for all integrin-mediated adhesion, acts in the same way in every context. We show that distinct combinations of talin domains are required for each of three different integrin functions during Drosophila development. The partial function of some mutant talins requires vinculin, indicating that recruitment of vinculin allows talin to duplicate its own activities. The different requirements are best explained by alternative mechanisms of talin function, with talin using one or both of its integrin-binding sites. We confirmed these alternatives by showing that the proximity between the second integrin-binding site and integrins differs, suggesting that talin adopts different orientations relative to integrins. Finally, we show that vinculin and actomyosin activity help change talin's orientation. These findings demonstrate that the mechanism of talin function differs in each developmental context examined. The different arrangements of the talin molecule relative to integrins suggest that talin is able to sense different force vectors, either parallel or perpendicular to the membrane. This provides a paradigm for proteins whose apparent uniform function is in fact achieved by a variety of distinct mechanisms involving different molecular architectures.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton.Nat Cell Biol. 2006 Jun;8(6):601-6. doi: 10.1038/ncb1411. Epub 2006 Apr 30. Nat Cell Biol. 2006. PMID: 16648844
-
Distinct developmental roles for direct and indirect talin-mediated linkage to actin.Dev Biol. 2010 Sep 1;345(1):64-77. doi: 10.1016/j.ydbio.2010.06.027. Epub 2010 Jun 28. Dev Biol. 2010. PMID: 20599891
-
Vinculin controls talin engagement with the actomyosin machinery.Nat Commun. 2015 Dec 4;6:10038. doi: 10.1038/ncomms10038. Nat Commun. 2015. PMID: 26634421 Free PMC article.
-
Talin - the master of integrin adhesions.J Cell Sci. 2017 Aug 1;130(15):2435-2446. doi: 10.1242/jcs.190991. Epub 2017 Jul 12. J Cell Sci. 2017. PMID: 28701514 Review.
-
Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion.Biochem Soc Trans. 2004 Nov;32(Pt 5):831-6. doi: 10.1042/BST0320831. Biochem Soc Trans. 2004. PMID: 15494027 Review.
Cited by
-
Vinculin recruitment to α-catenin halts the differentiation and maturation of enterocyte progenitors to maintain homeostasis of the Drosophila intestine.Elife. 2022 Oct 21;11:e72836. doi: 10.7554/eLife.72836. Elife. 2022. PMID: 36269226 Free PMC article.
-
An Integrated Stochastic Model of Matrix-Stiffness-Dependent Filopodial Dynamics.Biophys J. 2016 Nov 1;111(9):2051-2061. doi: 10.1016/j.bpj.2016.09.026. Biophys J. 2016. PMID: 27806285 Free PMC article.
-
The tale of two talins - two isoforms to fine-tune integrin signalling.FEBS Lett. 2018 Jun;592(12):2108-2125. doi: 10.1002/1873-3468.13081. Epub 2018 May 18. FEBS Lett. 2018. PMID: 29723415 Free PMC article. Review.
-
Diverse integrin adhesion stoichiometries caused by varied actomyosin activity.Open Biol. 2017 Apr;7(4):160250. doi: 10.1098/rsob.160250. Open Biol. 2017. PMID: 28446705 Free PMC article.
-
JNK signaling and integrins cooperate to maintain cell adhesion during epithelial fusion in Drosophila.Front Cell Dev Biol. 2024 Jan 9;11:1034484. doi: 10.3389/fcell.2023.1034484. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38264353 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

