The Induction of Pattern-Recognition Receptor Expression against Influenza A Virus through Duox2-Derived Reactive Oxygen Species in Nasal Mucosa
- PMID: 25751630
- PMCID: PMC5455469
- DOI: 10.1165/rcmb.2014-0334OC
The Induction of Pattern-Recognition Receptor Expression against Influenza A Virus through Duox2-Derived Reactive Oxygen Species in Nasal Mucosa
Abstract
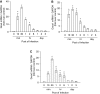
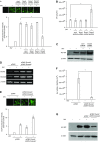
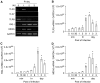
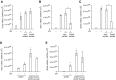
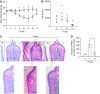
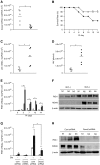
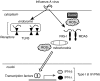
We studied the relative roles of Duox2-derived reactive oxygen species (ROS) in host defense against influenza A virus (IAV) infection in normal human nasal epithelial cells and mouse nasal mucosa. We found that Duox2 primarily generated ROS rapidly after IAV infection in normal human nasal epithelial cells and that knockdown of Duox2 aggravated IAV infection. In addition, Duox2-derived ROS enhancement significantly suppressed IAV infection in nasal epithelium. In particular, Duox2-derived ROS were required for the induction of retinoic acid-inducible gene (RIG)-I and melanoma differentiation-associated protein 5 (MDA5) transcription. After intranasal IAV inoculation into mice, viral infection was significantly aggravated from 3 days postinoculation (dpi) in the nasal mucosa, and the IAV viral titer was highest at 7 dpi. Both RIG-I and MDA5 messenger RNA levels increased dominantly in mouse nasal mucosa from 3 dpi; consistent with this, RIG-I and MDA5 proteins were also induced after IAV infection. RIG-I and MDA5 messenger RNA levels were induced to a lower extent in the nasal mucosa of the mice that were inoculated with Duox2 short hairpin RNA, and the IAV viral titer was significantly higher in nasal lavage. Taken together, Duox2-derived ROS are necessary for the innate immune response and trigger the induction of RIG-I and MDA5 to resist IAV infection in human nasal epithelium and mouse nasal mucosa.
Keywords: Duox2; influenza A virus; melanoma differentiation–associated protein 5; reactive oxygen species; retinoic acid–inducible gene-I.
Figures

Similar articles
-
Duox2 is required for the transcription of pattern recognition receptors in acute viral lung infection: An interferon-independent regulatory mechanism.Antiviral Res. 2016 Oct;134:1-5. doi: 10.1016/j.antiviral.2016.08.017. Epub 2016 Aug 18. Antiviral Res. 2016. PMID: 27546489
-
Duox2-induced innate immune responses in the respiratory epithelium and intranasal delivery of Duox2 DNA using polymer that mediates immunization.Appl Microbiol Biotechnol. 2018 May;102(10):4339-4343. doi: 10.1007/s00253-018-8956-y. Epub 2018 Mar 30. Appl Microbiol Biotechnol. 2018. PMID: 29600494 Review.
-
Intranasal delivery of Duox2 DNA using cationic polymer can prevent acute influenza A viral infection in vivo lung.Appl Microbiol Biotechnol. 2018 Jan;102(1):105-115. doi: 10.1007/s00253-017-8512-1. Epub 2017 Sep 21. Appl Microbiol Biotechnol. 2018. PMID: 28936773
-
Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells.Am J Respir Cell Mol Biol. 2013 Nov;49(5):855-65. doi: 10.1165/rcmb.2013-0003OC. Am J Respir Cell Mol Biol. 2013. PMID: 23786562 Free PMC article.
-
Cytoplasm and Beyond: Dynamic Innate Immune Sensing of Influenza A Virus by RIG-I.J Virol. 2019 Apr 3;93(8):e02299-18. doi: 10.1128/JVI.02299-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30760567 Free PMC article. Review.
Cited by
-
Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections.Oxid Med Cell Longev. 2022 Mar 8;2022:5589089. doi: 10.1155/2022/5589089. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35281470 Free PMC article. Review.
-
Exploring NAD+ metabolism in host-pathogen interactions.Cell Mol Life Sci. 2016 Mar;73(6):1225-36. doi: 10.1007/s00018-015-2119-4. Epub 2015 Dec 30. Cell Mol Life Sci. 2016. PMID: 26718485 Free PMC article. Review.
-
Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology.Int J Mol Sci. 2020 Dec 7;21(23):9317. doi: 10.3390/ijms21239317. Int J Mol Sci. 2020. PMID: 33297418 Free PMC article. Review.
-
Interplay between Inflammation and Cellular Stress Triggered by Flaviviridae Viruses.Front Microbiol. 2016 Aug 25;7:1233. doi: 10.3389/fmicb.2016.01233. eCollection 2016. Front Microbiol. 2016. PMID: 27610098 Free PMC article. Review.
-
Spatial Properties of Reactive Oxygen Species Govern Pathogen-Specific Immune System Responses.Antioxid Redox Signal. 2020 May 1;32(13):982-992. doi: 10.1089/ars.2020.8027. Epub 2020 Mar 6. Antioxid Redox Signal. 2020. PMID: 32008365 Free PMC article. Review.
References
-
- Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–344. - PubMed
-
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. - PubMed
-
- García-Sastre A, Biron CA. Type 1 interferons and the virus–host relationship: a lesson in détente. Science. 2006;312:879–882. - PubMed
-
- Wu S, Metcalf JP, Wu W. Innate immune response to influenza virus. Curr Opin Infect Dis. 2011;24:235–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

