Chitin and chitosan preparation from marine sources. Structure, properties and applications
- PMID: 25738328
- PMCID: PMC4377977
- DOI: 10.3390/md13031133
Chitin and chitosan preparation from marine sources. Structure, properties and applications
Abstract
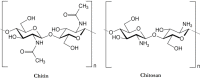
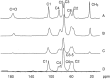
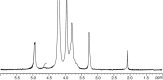
This review describes the most common methods for recovery of chitin from marine organisms. In depth, both enzymatic and chemical treatments for the step of deproteinization are compared, as well as different conditions for demineralization. The conditions of chitosan preparation are also discussed, since they significantly impact the synthesis of chitosan with varying degree of acetylation (DA) and molecular weight (MW). In addition, the main characterization techniques applied for chitin and chitosan are recalled, pointing out the role of their solubility in relation with the chemical structure (mainly the acetyl group distribution along the backbone). Biological activities are also presented, such as: antibacterial, antifungal, antitumor and antioxidant. Interestingly, the relationship between chemical structure and biological activity is demonstrated for chitosan molecules with different DA and MW and homogeneous distribution of acetyl groups for the first time. In the end, several selected pharmaceutical and biomedical applications are presented, in which chitin and chitosan are recognized as new biomaterials taking advantage of their biocompatibility and biodegradability.
Figures
Similar articles
-
Chilean crab (Aegla cholchol) as a new source of chitin and chitosan with antifungal properties against Candida spp.Int J Biol Macromol. 2020 Apr 15;149:962-975. doi: 10.1016/j.ijbiomac.2020.01.126. Epub 2020 Feb 8. Int J Biol Macromol. 2020. PMID: 32006582
-
Isolation and characterization of chitin and chitosan from marine origin.Adv Food Nutr Res. 2014;72:1-15. doi: 10.1016/B978-0-12-800269-8.00001-4. Adv Food Nutr Res. 2014. PMID: 25081074 Review.
-
Chitin, Characteristic, Sources, and Biomedical Application.Curr Pharm Biotechnol. 2020;21(14):1433-1443. doi: 10.2174/1389201021666200605104939. Curr Pharm Biotechnol. 2020. PMID: 32503407 Review.
-
Chitosan: A review of sources and preparation methods.Int J Biol Macromol. 2021 Feb 1;169:85-94. doi: 10.1016/j.ijbiomac.2020.12.005. Epub 2020 Dec 4. Int J Biol Macromol. 2021. PMID: 33279563 Review.
-
Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications.Int J Mol Sci. 2020 Jun 16;21(12):4290. doi: 10.3390/ijms21124290. Int J Mol Sci. 2020. PMID: 32560250 Free PMC article. Review.
Cited by
-
Turning Portunus pelagicus Shells into Biocompatible Scaffolds for Bone Regeneration.Biomedicines. 2024 Aug 7;12(8):1796. doi: 10.3390/biomedicines12081796. Biomedicines. 2024. PMID: 39200260 Free PMC article.
-
Nanoemulsions of red quinoa and ginseng extracts with chitosan wall: Investigating the antioxidant properties and its effect on the shelf life of dairy cream.Food Sci Nutr. 2024 May 21;12(8):5734-5749. doi: 10.1002/fsn3.4182. eCollection 2024 Aug. Food Sci Nutr. 2024. PMID: 39139958 Free PMC article.
-
Application of Polysaccharide Biopolymer in Petroleum Recovery.Polymers (Basel). 2020 Aug 19;12(9):1860. doi: 10.3390/polym12091860. Polymers (Basel). 2020. PMID: 32824986 Free PMC article. Review.
-
Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process.Sci Rep. 2022 Apr 22;12(1):6613. doi: 10.1038/s41598-022-10423-5. Sci Rep. 2022. PMID: 35459772 Free PMC article.
-
The Microfluidic Technique and the Manufacturing of Polysaccharide Nanoparticles.Pharmaceutics. 2018 Dec 9;10(4):267. doi: 10.3390/pharmaceutics10040267. Pharmaceutics. 2018. PMID: 30544868 Free PMC article. Review.
References
-
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. - DOI
-
- Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008;57:397–430.
-
- Rinaudo M. Physical properties of chitosan and derivatives in sol and gel states. In: Sarmento B., das Neves J., editors. Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics. John Wiley & Sons; Chichester, UK: 2012. pp. 23–44.
-
- Rinaudo M. Materials based on chitin and chitosan. In: Kabasci S., editor. Bio-Based Plastics: Materials and Applications. John Wiley & Sons; Chichester, UK: 2014. pp. 63–80.
-
- Rudall K.M., Kenchington W. The chitin system. Biol. Rev. 1973;40:597–636. doi: 10.1111/j.1469-185X.1973.tb01570.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

