GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration
- PMID: 25730668
- PMCID: PMC4734893
- DOI: 10.1038/nn.3966
GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration
Abstract
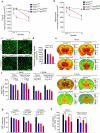
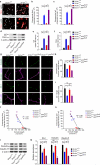
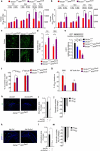
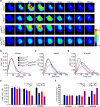
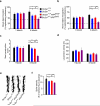
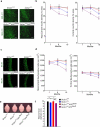
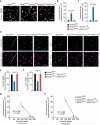
The glucose transporter GLUT1 at the blood-brain barrier (BBB) mediates glucose transport into the brain. Alzheimer's disease is characterized by early reductions in glucose transport associated with diminished GLUT1 expression at the BBB. Whether GLUT1 reduction influences disease pathogenesis remains, however, elusive. Here we show that GLUT1 deficiency in mice overexpressing amyloid β-peptide (Aβ) precursor protein leads to early cerebral microvascular degeneration, blood flow reductions and dysregulation and BBB breakdown, and to accelerated amyloid β-peptide (Aβ) pathology, reduced Aβ clearance, diminished neuronal activity, behavioral deficits, and progressive neuronal loss and neurodegeneration that develop after initial cerebrovascular degenerative changes. We also show that GLUT1 deficiency in endothelium, but not in astrocytes, initiates the vascular phenotype as shown by BBB breakdown. Thus, reduced BBB GLUT1 expression worsens Alzheimer's disease cerebrovascular degeneration, neuropathology and cognitive function, suggesting that GLUT1 may represent a therapeutic target for Alzheimer's disease vasculo-neuronal dysfunction and degeneration.
Figures
Comment in
-
Sugar and Alzheimer's disease: a bittersweet truth.Nat Neurosci. 2015 Apr;18(4):477-8. doi: 10.1038/nn.3986. Nat Neurosci. 2015. PMID: 25811474 Free PMC article.
Similar articles
-
ADAMTS13 maintains cerebrovascular integrity to ameliorate Alzheimer-like pathology.PLoS Biol. 2019 Jun 11;17(6):e3000313. doi: 10.1371/journal.pbio.3000313. eCollection 2019 Jun. PLoS Biol. 2019. Retraction in: PLoS Biol. 2023 Mar 9;21(3):e3002036. doi: 10.1371/journal.pbio.3002036 PMID: 31185010 Free PMC article. Retracted.
-
Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice.Acta Neuropathol. 2011 Sep;122(3):293-311. doi: 10.1007/s00401-011-0834-y. Epub 2011 Jun 19. Acta Neuropathol. 2011. PMID: 21688176 Free PMC article.
-
Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer's disease.J Alzheimers Dis. 2013;33 Suppl 1(0 1):S87-100. doi: 10.3233/JAD-2012-129037. J Alzheimers Dis. 2013. PMID: 22751174 Free PMC article. Review.
-
Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease.Cold Spring Harb Perspect Med. 2012 Oct 1;2(10):a011452. doi: 10.1101/cshperspect.a011452. Cold Spring Harb Perspect Med. 2012. PMID: 23028132 Free PMC article. Review.
-
Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease.Acta Neuropathol. 2009 Jul;118(1):103-13. doi: 10.1007/s00401-009-0522-3. Epub 2009 Mar 25. Acta Neuropathol. 2009. PMID: 19319544 Free PMC article. Review.
Cited by
-
Postnatal Maturation of the Blood-Brain Barrier in Senescence-Accelerated OXYS Rats, Which Are Prone to an Alzheimer's Disease-like Pathology.Int J Mol Sci. 2023 Oct 27;24(21):15649. doi: 10.3390/ijms242115649. Int J Mol Sci. 2023. PMID: 37958635 Free PMC article.
-
D-Glucose uptake and clearance in the tauopathy Alzheimer's disease mouse brain detected by on-resonance variable delay multiple pulse MRI.J Cereb Blood Flow Metab. 2021 May;41(5):1013-1025. doi: 10.1177/0271678X20941264. Epub 2020 Jul 16. J Cereb Blood Flow Metab. 2021. PMID: 32669023 Free PMC article.
-
Remodeling of the Neurovascular Unit Following Cerebral Ischemia and Hemorrhage.Cells. 2022 Sep 9;11(18):2823. doi: 10.3390/cells11182823. Cells. 2022. PMID: 36139398 Free PMC article. Review.
-
Identification of Blood Biomarkers Related to Energy Metabolism and Construction of Diagnostic Prediction Model Based on Three Independent Alzheimer's Disease Cohorts.J Alzheimers Dis. 2024;100(4):1261-1287. doi: 10.3233/JAD-240301. J Alzheimers Dis. 2024. PMID: 39093073 Free PMC article.
-
Ketogenic Diet in Alzheimer's Disease.Int J Mol Sci. 2019 Aug 9;20(16):3892. doi: 10.3390/ijms20163892. Int J Mol Sci. 2019. PMID: 31405021 Free PMC article. Review.
References
-
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. - PubMed
-
- Deng D, et al. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121–125. - PubMed
-
- Allen A, Messier C. Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav. Brain Res. 2013;240:95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

