Statistical considerations for preclinical studies
- PMID: 25725352
- PMCID: PMC4466166
- DOI: 10.1016/j.expneurol.2015.02.024
Statistical considerations for preclinical studies
Abstract
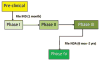
Research studies must always have proper planning, conduct, analysis and reporting in order to preserve scientific integrity. Preclinical studies, the first stage of the drug development process, are no exception to this rule. The decision to advance to clinical trials in humans relies on the results of these studies. Recent observations show that a significant number of preclinical studies lack rigor in their conduct and reporting. This paper discusses statistical aspects, such as design, sample size determination, and methods of analyses, that will help add rigor and improve the quality of preclinical studies.
Keywords: False positive; Missing data; Multiple outcomes; Power; Preclinical studies; Randomization; Sample size.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Standards and Methodological Rigor in Pulmonary Arterial Hypertension Preclinical and Translational Research.Circ Res. 2018 Mar 30;122(7):1021-1032. doi: 10.1161/CIRCRESAHA.117.312579. Circ Res. 2018. PMID: 29599278 Review.
-
Methodological Rigor in Preclinical Cardiovascular Studies: Targets to Enhance Reproducibility and Promote Research Translation.Circ Res. 2017 Jun 9;120(12):1916-1926. doi: 10.1161/CIRCRESAHA.117.310628. Epub 2017 Apr 3. Circ Res. 2017. PMID: 28373349 Free PMC article. Review.
-
Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation.J Cardiovasc Pharmacol Ther. 2011 Sep-Dec;16(3-4):332-9. doi: 10.1177/1074248411414155. J Cardiovasc Pharmacol Ther. 2011. PMID: 21821536
-
Design and Conduct Considerations for First-in-Human Trials.Clin Transl Sci. 2019 Jan;12(1):6-19. doi: 10.1111/cts.12582. Epub 2018 Aug 24. Clin Transl Sci. 2019. PMID: 30048046 Free PMC article.
-
Use and abuse of QT and TRIaD in cardiac safety research: importance of study design and conduct.Eur J Pharmacol. 2008 Apr 14;584(1):1-9. doi: 10.1016/j.ejphar.2008.01.016. Epub 2008 Jan 26. Eur J Pharmacol. 2008. PMID: 18304526 Review.
Cited by
-
Feasibility study of intraoperative pericardial fluid biomarkers and length of stay after cardiac surgery.JTCVS Tech. 2023 Apr 14;19:86-92. doi: 10.1016/j.xjtc.2023.03.018. eCollection 2023 Jun. JTCVS Tech. 2023. PMID: 37324339 Free PMC article.
-
Data Management Schema Design for Effective Nanoparticle Formulation for Neurotherapeutics.AIChE J. 2021 Dec;67(12):e17459. doi: 10.1002/aic.17459. Epub 2021 Sep 22. AIChE J. 2021. PMID: 35399334 Free PMC article.
-
Statistical practice and transparent reporting in the neurosciences: Preclinical motor behavioral experiments.PLoS One. 2022 Mar 21;17(3):e0265154. doi: 10.1371/journal.pone.0265154. eCollection 2022. PLoS One. 2022. PMID: 35312695 Free PMC article.
-
Mouse Models of Lung Fibrosis.Methods Mol Biol. 2021;2299:291-321. doi: 10.1007/978-1-0716-1382-5_21. Methods Mol Biol. 2021. PMID: 34028751
-
Urokinase-type plasminogen activator promotes N-cadherin-mediated synaptic recovery in the ischemic brain.J Cereb Blood Flow Metab. 2021 Sep;41(9):2381-2394. doi: 10.1177/0271678X211002297. Epub 2021 Mar 24. J Cereb Blood Flow Metab. 2021. PMID: 33757316 Free PMC article.
References
-
- Agresti A. An Introduction to Categorical Data Analysis. 2. John Wiley and Sons; NJ: 2007.
-
- Albert PS. Longitudinal Data Analysis (Repeated Measures) in Clinical Trials. Statistics in Medicine. 1999;18:1707–1732. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300.
-
- Goodman S. A dirty dozen: twelve p-value misconceptions. Seminars in Hematology. 2008;45:135–140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources