Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB
- PMID: 25720963
- PMCID: PMC4801004
- DOI: 10.1038/ncb3114
Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB
Abstract
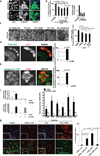
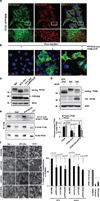
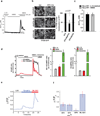
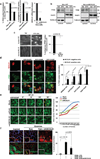
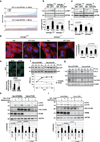
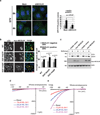
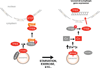
The view of the lysosome as the terminal end of cellular catabolic pathways has been challenged by recent studies showing a central role of this organelle in the control of cell function. Here we show that a lysosomal Ca2+ signalling mechanism controls the activities of the phosphatase calcineurin and of its substrate TFEB, a master transcriptional regulator of lysosomal biogenesis and autophagy. Lysosomal Ca2+ release through mucolipin 1 (MCOLN1) activates calcineurin, which binds and dephosphorylates TFEB, thus promoting its nuclear translocation. Genetic and pharmacological inhibition of calcineurin suppressed TFEB activity during starvation and physical exercise, while calcineurin overexpression and constitutive activation had the opposite effect. Induction of autophagy and lysosomal biogenesis through TFEB required MCOLN1-mediated calcineurin activation. These data link lysosomal calcium signalling to both calcineurin regulation and autophagy induction and identify the lysosome as a hub for the signalling pathways that regulate cellular homeostasis.
Figures

Comment in
-
Intracellular calcium signaling regulates autophagy via calcineurin-mediated TFEB dephosphorylation.Autophagy. 2015;11(7):1192-5. doi: 10.1080/15548627.2015.1054594. Autophagy. 2015. PMID: 26043755 Free PMC article.
Similar articles
-
Intracellular calcium signaling regulates autophagy via calcineurin-mediated TFEB dephosphorylation.Autophagy. 2015;11(7):1192-5. doi: 10.1080/15548627.2015.1054594. Autophagy. 2015. PMID: 26043755 Free PMC article.
-
Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration.Autophagy. 2019 Apr;15(4):631-651. doi: 10.1080/15548627.2018.1535292. Epub 2018 Nov 5. Autophagy. 2019. PMID: 30335591 Free PMC article.
-
Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity.Cell Death Dis. 2018 Dec 13;9(12):1191. doi: 10.1038/s41419-018-1227-0. Cell Death Dis. 2018. PMID: 30546014 Free PMC article.
-
Lysosomal calcium regulates autophagy.Autophagy. 2015;11(6):970-1. doi: 10.1080/15548627.2015.1047130. Autophagy. 2015. PMID: 26000950 Free PMC article. Review.
-
Pancreatic β-cell mitophagy as an adaptive response to metabolic stress and the underlying mechanism that involves lysosomal Ca2+ release.Exp Mol Med. 2023 Sep;55(9):1922-1932. doi: 10.1038/s12276-023-01055-4. Epub 2023 Sep 1. Exp Mol Med. 2023. PMID: 37653033 Free PMC article. Review.
Cited by
-
PDCD4 triggers α-synuclein accumulation and motor deficits via co-suppressing TFE3 and TFEB translation in a model of Parkinson's disease.NPJ Parkinsons Dis. 2024 Aug 6;10(1):146. doi: 10.1038/s41531-024-00760-9. NPJ Parkinsons Dis. 2024. PMID: 39107320 Free PMC article.
-
Molecular Mechanisms of Lysosome and Nucleus Communication.Trends Biochem Sci. 2020 Nov;45(11):978-991. doi: 10.1016/j.tibs.2020.06.004. Epub 2020 Jul 2. Trends Biochem Sci. 2020. PMID: 32624271 Free PMC article. Review.
-
Regulation of longevity by depolarization-induced activation of PLC-β-IP3R signaling in neurons.Proc Natl Acad Sci U S A. 2021 Apr 20;118(16):e2004253118. doi: 10.1073/pnas.2004253118. Proc Natl Acad Sci U S A. 2021. PMID: 33859040 Free PMC article.
-
The Cryptococcus neoformans Flc1 Homologue Controls Calcium Homeostasis and Confers Fungal Pathogenicity in the Infected Hosts.mBio. 2022 Oct 26;13(5):e0225322. doi: 10.1128/mbio.02253-22. Epub 2022 Sep 28. mBio. 2022. PMID: 36169198 Free PMC article.
-
Drug Sequestration in Lysosomes as One of the Mechanisms of Chemoresistance of Cancer Cells and the Possibilities of Its Inhibition.Int J Mol Sci. 2020 Jun 20;21(12):4392. doi: 10.3390/ijms21124392. Int J Mol Sci. 2020. PMID: 32575682 Free PMC article. Review.
References
-
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. - PubMed
-
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nature Rev. Mol. Cell Biol. 2009;10:623–635. - PubMed
-
- de Duve C. The lysosome turns fifty. Nature Cell Biol. 2005;7:847–849. - PubMed
-
- Luzio JP, Parkinson MD, Gray SR, Bright NA. The delivery of endocytosed cargo to lysosomes. Biochem. Soc. Trans. 2009;37:1019–1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

