Correlation of disease activity in proliferative glomerulonephritis with glomerular spleen tyrosine kinase expression
- PMID: 25715120
- PMCID: PMC4488852
- DOI: 10.1038/ki.2015.29
Correlation of disease activity in proliferative glomerulonephritis with glomerular spleen tyrosine kinase expression
Abstract
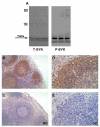
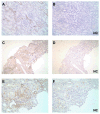
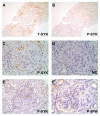
Spleen tyrosine kinase (SYK) is an important component of the intracellular signaling pathway for various immunoreceptors. Inhibition of SYK has shown promise in preclinical models of autoimmune and glomerular disease. However, the description of SYK expression in human renal tissue, which would be desirable ahead of clinical studies, is lacking. Here we conducted immunohistochemical analysis for total and phosphorylated SYK in biopsy specimens from >120 patients with a spectrum of renal pathologies, including thin basement membrane lesion, minimal change disease, membranous nephropathy, IgA nephropathy, lupus nephritis, ANCA-associated glomerulonephritis, antiglomerular basement membrane disease, and acute tubular necrosis. We found significant SYK expression in proliferative glomerulonephritis and that glomerular expression levels correlated with presenting serum creatinine and histological features of disease activity that predict outcome in IgA nephropathy, lupus nephritis, ANCA-associated glomerulonephritis, and antiglomerular basement membrane disease. SYK was phosphorylated within pathological lesions, such as areas of extracapillary and endocapillary proliferation, and appeared to localize to both infiltrating leucocytes and to resident renal cells within diseased glomeruli. Thus SYK is associated with the pathogenesis of proliferative glomerulonephritides, suggesting that these conditions may respond to SYK inhibitor treatment.
Figures
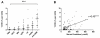





Similar articles
-
Myeloid cell-mediated renal injury in rapidly progressive glomerulonephritis depends upon spleen tyrosine kinase.J Pathol. 2016 Jan;238(1):10-20. doi: 10.1002/path.4598. Epub 2015 Sep 22. J Pathol. 2016. PMID: 26251216
-
Spleen tyrosine kinase promotes acute neutrophil-mediated glomerular injury via activation of JNK and p38 MAPK in rat nephrotoxic serum nephritis.Lab Invest. 2011 Dec;91(12):1727-38. doi: 10.1038/labinvest.2011.137. Epub 2011 Sep 5. Lab Invest. 2011. PMID: 21894146
-
An inhibitor of spleen tyrosine kinase suppresses experimental crescentic glomerulonephritis.Int J Immunopathol Pharmacol. 2018 Jan-Dec;32:2058738418783404. doi: 10.1177/2058738418783404. Int J Immunopathol Pharmacol. 2018. PMID: 29923438 Free PMC article.
-
Role of the Spleen Tyrosine Kinase Pathway in Driving Inflammation in IgA Nephropathy.Semin Nephrol. 2018 Sep;38(5):496-503. doi: 10.1016/j.semnephrol.2018.05.019. Semin Nephrol. 2018. PMID: 30177021 Free PMC article. Review.
-
Targeting spleen tyrosine kinase-Bruton's tyrosine kinase axis for immunologically mediated glomerulonephritis.Biomed Res Int. 2014;2014:814869. doi: 10.1155/2014/814869. Epub 2014 Mar 30. Biomed Res Int. 2014. PMID: 24795896 Free PMC article. Review.
Cited by
-
Renal macrophages and NLRP3 inflammasomes in kidney diseases and therapeutics.Cell Death Discov. 2024 May 13;10(1):229. doi: 10.1038/s41420-024-01996-3. Cell Death Discov. 2024. PMID: 38740765 Free PMC article. Review.
-
An Update on the Current State of Management and Clinical Trials for IgA Nephropathy.J Clin Med. 2021 Jun 4;10(11):2493. doi: 10.3390/jcm10112493. J Clin Med. 2021. PMID: 34200024 Free PMC article. Review.
-
Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: from bench to bedside and beyond.Nephrol Dial Transplant. 2017 Jan 1;32(suppl_1):i129-i138. doi: 10.1093/ndt/gfw336. Nephrol Dial Transplant. 2017. PMID: 28391340 Free PMC article. Review.
-
Randomized Trial on the Effect of an Oral Spleen Tyrosine Kinase Inhibitor in the Treatment of IgA Nephropathy.Kidney Int Rep. 2023 Sep 30;8(12):2546-2556. doi: 10.1016/j.ekir.2023.09.024. eCollection 2023 Dec. Kidney Int Rep. 2023. PMID: 38106605 Free PMC article.
-
New insights into the pathogenesis of IgA nephropathy.Pediatr Nephrol. 2018 May;33(5):763-777. doi: 10.1007/s00467-017-3699-z. Epub 2017 Jun 17. Pediatr Nephrol. 2018. PMID: 28624979 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

