Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde
- PMID: 25712056
- PMCID: PMC4417412
- DOI: 10.1158/1940-6207.CAPR-14-0359
Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde
Abstract
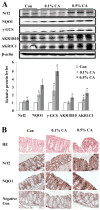
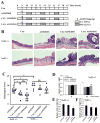
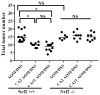
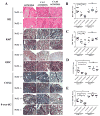
The progressive nature of colorectal cancer and poor prognosis associated with the metastatic phase of the disease create an urgent need for the development of more efficacious strategies targeting colorectal carcinogenesis. Cumulative evidence suggests that the redox-sensitive transcription factor Nrf2 (nuclear factor-E2-related factor 2), a master regulator of the cellular antioxidant defence, represents a promising molecular target for colorectal cancer chemoprevention. Recently, we have identified cinnamon, the ground bark of Cinnamomum aromaticum (cassia cinnamon) and Cinnamomum verum (Ceylon cinnamon), as a rich dietary source of the Nrf2 inducer cinnamaldehyde (CA) eliciting the Nrf2-regulated antioxidant response in human epithelial colon cells, conferring cytoprotection against electrophilic and genotoxic insult. Here, we have explored the molecular mechanism underlying CA-induced Nrf2 activation in colorectal epithelial cells and have examined the chemopreventive potential of CA in a murine colorectal cancer model comparing Nrf2(+/+) with Nrf2(-/-) mice. In HCT116 cells, CA caused a Keap1-C151-dependent increase in Nrf2 protein half-life via blockage of ubiquitination with upregulation of cytoprotective Nrf2 target genes and elevation of cellular glutathione. After optimizing colorectal Nrf2 activation and target gene expression by dietary CA-supplementation regimens, we demonstrated that CA suppresses AOM/DSS-induced inflammatory colon carcinogenesis with modulation of molecular markers of colorectal carcinogenesis. Dietary suppression of colorectal cancer using CA supplementation was achieved in Nrf2(+/+) but not in Nrf2(-/-) mice confirming the Nrf2 dependence of CA-induced chemopreventive effects. Taken together, our data suggest feasibility of colorectal cancer suppression by dietary CA, an FDA-approved food additive derived from the third most consumed spice in the world.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures

Similar articles
-
The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells.Molecules. 2010 May 7;15(5):3338-55. doi: 10.3390/molecules15053338. Molecules. 2010. PMID: 20657484 Free PMC article.
-
Identification of novel Nrf2 target genes as prognostic biomarkers in colitis-associated colorectal cancer in Nrf2-deficient mice.Life Sci. 2019 Dec 1;238:116968. doi: 10.1016/j.lfs.2019.116968. Epub 2019 Oct 16. Life Sci. 2019. PMID: 31628914
-
Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer.Cancer Prev Res (Phila). 2008 Aug;1(3):187-91. doi: 10.1158/1940-6207.CAPR-08-0028. Epub 2008 Mar 31. Cancer Prev Res (Phila). 2008. PMID: 19138955 Free PMC article.
-
Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals.Toxicol Lett. 2014 Aug 17;229(1):73-84. doi: 10.1016/j.toxlet.2014.05.018. Epub 2014 May 27. Toxicol Lett. 2014. PMID: 24875534 Review.
-
Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells.Food Chem Toxicol. 2008 Apr;46(4):1257-70. doi: 10.1016/j.fct.2007.09.082. Epub 2007 Sep 15. Food Chem Toxicol. 2008. PMID: 17950513 Review.
Cited by
-
Antioxidant and Anti-Apoptotic Neuroprotective Effects of Cinnamon in Imiquimod-Induced Lupus.Antioxidants (Basel). 2024 Jul 22;13(7):880. doi: 10.3390/antiox13070880. Antioxidants (Basel). 2024. PMID: 39061948 Free PMC article.
-
Metformin Activates the AMPK-mTOR Pathway by Modulating lncRNA TUG1 to Induce Autophagy and Inhibit Atherosclerosis.Drug Des Devel Ther. 2020 Feb 3;14:457-468. doi: 10.2147/DDDT.S233932. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32099330 Free PMC article.
-
NRF2 and the Ambiguous Consequences of Its Activation during Initiation and the Subsequent Stages of Tumourigenesis.Cancers (Basel). 2020 Dec 2;12(12):3609. doi: 10.3390/cancers12123609. Cancers (Basel). 2020. PMID: 33276631 Free PMC article. Review.
-
The molecular biology and therapeutic potential of Nrf2 in leukemia.Cancer Cell Int. 2022 Jul 29;22(1):241. doi: 10.1186/s12935-022-02660-5. Cancer Cell Int. 2022. PMID: 35906617 Free PMC article. Review.
-
NRF2 and the Hallmarks of Cancer.Cancer Cell. 2018 Jul 9;34(1):21-43. doi: 10.1016/j.ccell.2018.03.022. Epub 2018 May 3. Cancer Cell. 2018. PMID: 29731393 Free PMC article. Review.
References
-
- Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–9. - PubMed
-
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA023074/CA/NCI NIH HHS/United States
- 2R01ES015010/ES/NIEHS NIH HHS/United States
- R03 CA167580/CA/NCI NIH HHS/United States
- T32 ES007091/ES/NIEHS NIH HHS/United States
- ES06694/ES/NIEHS NIH HHS/United States
- P30 ES006694/ES/NIEHS NIH HHS/United States
- R21CA166926/CA/NCI NIH HHS/United States
- CA023074/CA/NCI NIH HHS/United States
- R01 CA154377/CA/NCI NIH HHS/United States
- R21 CA166926/CA/NCI NIH HHS/United States
- ES007091/ES/NIEHS NIH HHS/United States
- R01CA154377/CA/NCI NIH HHS/United States
- R01 ES015010/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources

