Challenges, successes and patterns of enrolment in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial
- PMID: 25711319
- PMCID: PMC4373412
- DOI: 10.1111/hiv.12229
Challenges, successes and patterns of enrolment in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial
Abstract
Objectives: The aim of this report is to describe the challenges, successes and patterns of enrolment in the Strategic Timing of AntiRetroviral Treatment (START) study.
Methods: START is a collaboration of many partners with central coordination provided by the protocol team, the statistical and data management centre (SDMC), the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) network leadership, international coordinating centres and site coordinating centres. The SDMC prepared reports on study accrual, baseline characteristics and site performance that allowed monitoring of enrolment and data quality and helped to ensure the successful enrolment of this large international trial. We describe the pattern of enrolment and challenges faced during the enrolment period of the trial.
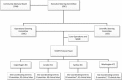
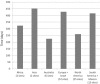
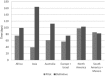
Results: An initial pilot phase began in April 2009 and established feasibility of accrual at 101 sites. In August 2010, funding approval for an expanded definitive phase led to the successful accrual of 4688 participants from 215 sites in 35 countries by December 2013. Challenges to accrual included regulatory delays (e.g. national/local ethics approval and drug importation approval) and logistical obstacles (e.g. execution of contracts with pharmaceutical companies, setting up of a central drug repository and translation of participant materials). The personal engagement of investigators, strong central study coordination, and frequent and transparent communication with site investigators, community members and participants were key contributing factors to this success.
Conclusions: Accrual into START was completed in a timely fashion despite multiple challenges. This success was attributable to the efforts of site investigators committed to maintaining study equipoise, transparent and responsive study coordination, and community involvement in problem-solving.
Trial registration: ClinicalTrials.gov NCT00867048.
Keywords: HIV/AIDS; START trial; antiretroviral therapy initiation; enrolment.
© 2015 British HIV Association.
Figures

Similar articles
-
Demographic and HIV-specific characteristics of participants enrolled in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial.HIV Med. 2015 Apr;16 Suppl 1(0 1):30-6. doi: 10.1111/hiv.12231. HIV Med. 2015. PMID: 25711321 Free PMC article. Clinical Trial.
-
Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study.Clin Trials. 2013;10(1 Suppl):S5-S36. doi: 10.1177/1740774512440342. Epub 2012 Apr 30. Clin Trials. 2013. PMID: 22547421 Free PMC article. Clinical Trial.
-
Community perspective on the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial.HIV Med. 2015 Apr;16 Suppl 1(0 1):10-3. doi: 10.1111/hiv.12228. HIV Med. 2015. PMID: 25711318 Free PMC article. Clinical Trial.
-
Antiretroviral therapy: when to start.Infect Dis Clin North Am. 2014 Sep;28(3):403-20. doi: 10.1016/j.idc.2014.05.004. Infect Dis Clin North Am. 2014. PMID: 25151563 Free PMC article. Review.
-
Antiretroviral Therapy for HIV Infection: When to Initiate Therapy, Which Regimen to Use, and How to Monitor Patients on Therapy.Top Antivir Med. 2016 Dec-2017 Jan;23(5):161-7. Top Antivir Med. 2016. PMID: 27398769 Free PMC article. Review.
Cited by
-
Residual immune activation in HIV-Infected individuals expands monocytic-myeloid derived suppressor cells.Cell Immunol. 2021 Apr;362:104304. doi: 10.1016/j.cellimm.2021.104304. Epub 2021 Feb 10. Cell Immunol. 2021. PMID: 33610024 Free PMC article.
-
Operational complexities in international clinical trials: a systematic review of challenges and proposed solutions.BMJ Open. 2024 Apr 15;14(4):e077132. doi: 10.1136/bmjopen-2023-077132. BMJ Open. 2024. PMID: 38626966 Free PMC article.
-
Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection.N Engl J Med. 2015 Aug 27;373(9):795-807. doi: 10.1056/NEJMoa1506816. Epub 2015 Jul 20. N Engl J Med. 2015. PMID: 26192873 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

