Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial
- PMID: 25704439
- PMCID: PMC5726228
- DOI: 10.1016/S1470-2045(15)70054-9
Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial
Abstract
Background: Patients with squamous non-small-cell lung cancer that is refractory to multiple treatments have poor outcomes. We assessed the activity of nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor antibody, for patients with advanced, refractory, squamous non-small-cell lung cancer.
Methods: We did this phase 2, single-arm trial at 27 sites (academic, hospital, and private cancer centres) in France, Germany, Italy, and USA. Patients who had received two or more previous treatments received intravenous nivolumab (3 mg/kg) every 2 weeks until progression or unacceptable toxic effects. The primary endpoint was the proportion of patients with a confirmed objective response as assessed by an independent radiology review committee. We included all treated patients in the analyses. This study is registered with ClinicalTrials.gov, number NCT01721759.
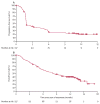
Findings: Between Nov 16, 2012, and July 22, 2013, we enrolled and treated 117 patients. 17 (14·5%, 95% CI 8·7-22·2) of 117 patients had an objective response as assessed by an independent radiology review committee. Median time to response was 3·3 months (IQR 2·2-4·8), and median duration of response was not reached (95% CI 8·31-not applicable); 13 (77%) of 17 of responses were ongoing at the time of analysis. 30 (26%) of 117 patients had stable disease (median duration 6·0 months, 95% CI 4·7-10·9). 20 (17%) of 117 patients reported grade 3-4 treatment-related adverse events, including: fatigue (five [4%] of 117 patients), pneumonitis (four [3%]), and diarrhoea (three [3%]). There were two treatment-associated deaths caused by pneumonia and ischaemic stroke that occurred in patients with multiple comorbidities in the setting of progressive disease.
Interpretation: Nivolumab has clinically meaningful activity and a manageable safety profile in previously treated patients with advanced, refractory, squamous non-small cell lung cancer. These data support the assessment of nivolumab in randomised, controlled, phase 3 studies of first-line and second-line treatment.
Funding: Bristol-Myers Squibb.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
NAR has received personal fees from Bristol-Myers Squibb, Genentech, Roche, MedImmune, AstraZeneca, and Merck. TES has received research grant support from Bristol-Myers Squibb and personal fees from Genentech, Eli Lilly, Celgene, and Boehringer Ingelheim. SJA has received research grant support from MedImmune, and personal fees from Bristol-Myers Squibb, MedImmune, and AstraZeneca. LH has received research grant support from Astellas, has served as a non-paid consultant to Bayer and Xcovery, and has received personal fees from Bristol-Myers Squibb, Merck, Clovis, Helix Bio, and Genentech. HL has received personal fees from Bristol-Myers Squibb and Merck, and non-financial support from Bristol-Myers Squibb and Roche. BM has served as a consultant to Bristol-Myers Squibb and on advisory boards for Merck Sharp & Dohme. GAO has received research grant support from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, New Link Genetics, Genentech, and Boehringer Ingelheim, and has received personal fees from Genentech and Boehringer Ingelheim. DRG has received research grant support from Bristol-Myers Squibb. BPL has received personal fees from Eli Lilly, Genentech, Pfizer, Biodesix, and Boehringer Ingelheim. GZ has received personal fees from Bristol-Myers Squibb. JW has received research grants from Boehringer Ingelheim, Novartis, Pfizer, Roche, and Bayer, and has served on advisory boards for and received lecture fees from Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Clovis, Novartis, Pfizer, Roche, and Merck Sharp and Dohme. PJS has received personal fees, non-financial support, and support for clinical studies from Roche. FC has received personal fees from Bristol-Myers Squibb. CC has served as a consultant to Bristol-Myers Squibb, Merck, and Roche. RS has received research grant support and honoraria from and has served on advisory boards for Bristol-Myers Squibb. RMH has served on advisory boards for Bristol-Myers Squibb. CTH, CB, and BJL are employed by and own stock in Bristol-Myers Squibb. SSR has received research grant support and personal fees from Bristol-Myers Squibb and has received personal fees from Amgen, AstraZeneca, Aveo, Boehringer Ingelheim, Celgene, Genentech, Eli Lilly, and Novartis. SJA, DRG, AD, and SSR have received funding from the National Institutes of Health (USA). The other authors declare no competing interests.
Figures
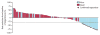

Comment in
-
Nivolumab for advanced squamous cell lung cancer: what are the next steps?Lancet Oncol. 2015 Mar;16(3):234-5. doi: 10.1016/S1470-2045(15)70074-4. Epub 2015 Feb 20. Lancet Oncol. 2015. PMID: 25704436 No abstract available.
Similar articles
-
Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2019 Nov;20(11):1506-1517. doi: 10.1016/S1470-2045(19)30626-6. Epub 2019 Sep 30. Lancet Oncol. 2019. PMID: 31582355 Clinical Trial.
-
Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer.N Engl J Med. 2015 Jul 9;373(2):123-35. doi: 10.1056/NEJMoa1504627. Epub 2015 May 31. N Engl J Med. 2015. PMID: 26028407 Free PMC article. Clinical Trial.
-
FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy.Oncologist. 2016 May;21(5):634-42. doi: 10.1634/theoncologist.2015-0507. Epub 2016 Mar 16. Oncologist. 2016. PMID: 26984449 Free PMC article. Clinical Trial.
-
Nivolumab: a review in advanced squamous non-small cell lung cancer.Drugs. 2015 Nov;75(16):1925-34. doi: 10.1007/s40265-015-0492-9. Drugs. 2015. PMID: 26514815 Review.
-
Nivolumab: A Review in Advanced Melanoma.Drugs. 2015 Aug;75(12):1413-24. doi: 10.1007/s40265-015-0442-6. Drugs. 2015. PMID: 26220912 Review.
Cited by
-
Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non-Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01).J Clin Oncol. 2023 Jan 20;41(3):651-663. doi: 10.1200/JCO.22.00727. Epub 2022 Oct 7. J Clin Oncol. 2023. PMID: 36206498 Free PMC article. Clinical Trial.
-
Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC).J Neurooncol. 2016 Oct;130(1):19-29. doi: 10.1007/s11060-016-2216-8. Epub 2016 Jul 19. J Neurooncol. 2016. PMID: 27436101
-
Primary Resistance to Immune Checkpoint Blockade in an STK11/TP53/KRAS-Mutant Lung Adenocarcinoma with High PD-L1 Expression.Onco Targets Ther. 2020 Sep 7;13:8901-8905. doi: 10.2147/OTT.S272013. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982282 Free PMC article.
-
Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer.J Clin Oncol. 2016 Sep 1;34(25):2980-7. doi: 10.1200/JCO.2016.66.9929. Epub 2016 Jun 27. J Clin Oncol. 2016. PMID: 27354485 Free PMC article. Clinical Trial.
-
Destabilizing the genome as a therapeutic strategy to enhance response to immune checkpoint blockade: a systematic review of clinical trials evidence from solid and hematological tumors.Front Pharmacol. 2024 Jan 9;14:1280591. doi: 10.3389/fphar.2023.1280591. eCollection 2023. Front Pharmacol. 2024. PMID: 38264532 Free PMC article.
References
-
- Domingues D, Turner A, Dilla Silva M. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy. 2014;6:1221–35. - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

