Managing population immunity to reduce or eliminate the risks of circulation following the importation of polioviruses
- PMID: 25701673
- PMCID: PMC7907970
- DOI: 10.1016/j.vaccine.2015.02.013
Managing population immunity to reduce or eliminate the risks of circulation following the importation of polioviruses
Abstract
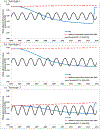
Poliovirus importations into polio-free countries represent a major concern during the final phases of global eradication of wild polioviruses (WPVs). We extend dynamic transmission models to demonstrate the dynamics of population immunity out through 2020 for three countries that only used inactivated poliovirus vaccine (IPV) for routine immunization: the US, Israel, and The Netherlands. For each country, we explore the vulnerability to re-established transmission following an importation for each poliovirus serotype, including the impact of immunization choices following the serotype 1 WPV importation that occurred in 2013 in Israel. As population immunity declines below the threshold required to prevent transmission, countries become at risk for re-established transmission. Although importations represent stochastic events that countries cannot fully control because people cross borders and polioviruses mainly cause asymptomatic infections, countries can ensure that any importations die out. Our results suggest that the general US population will remain above the threshold for transmission through 2020. In contrast, Israel became vulnerable to re-established transmission of importations of live polioviruses by the late 2000s. In Israel, the recent WPV importation and outbreak response use of bivalent oral poliovirus vaccine (bOPV) eliminated the vulnerability to an importation of poliovirus serotypes 1 and 3 for several years, but not serotype 2. The Netherlands experienced a serotype 1 WPV outbreak in 1992-1993 and became vulnerable to re-established transmission in religious communities with low vaccine acceptance around the year 2000, although the general population remains well-protected from widespread transmission. All countries should invest in active management of population immunity to avoid the potential circulation of imported live polioviruses. IPV-using countries may wish to consider prevention opportunities and/or ensure preparedness for response. Countries currently using a sequential IPV/OPV schedule should continue to use all licensed OPV serotypes until global OPV cessation to minimize vulnerability to circulation of imported polioviruses.
Keywords: Eradication; Polio; Population immunity; Vaccine.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Figures





Similar articles
-
Planning for globally coordinated cessation of bivalent oral poliovirus vaccine: risks of non-synchronous cessation and unauthorized oral poliovirus vaccine use.BMC Infect Dis. 2018 Apr 10;18(1):165. doi: 10.1186/s12879-018-3074-0. BMC Infect Dis. 2018. PMID: 29631539 Free PMC article.
-
Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of potential non-synchronous cessation.BMC Infect Dis. 2016 May 26;16:231. doi: 10.1186/s12879-016-1536-9. BMC Infect Dis. 2016. PMID: 27230071 Free PMC article.
-
Modeling options to manage type 1 wild poliovirus imported into Israel in 2013.J Infect Dis. 2015 Jun 1;211(11):1800-12. doi: 10.1093/infdis/jiu674. Epub 2014 Dec 10. J Infect Dis. 2015. PMID: 25505296 Free PMC article.
-
Trade-offs of different poliovirus vaccine options for outbreak response in the United States and other countries that only use inactivated poliovirus vaccine (IPV) in routine immunization.Vaccine. 2024 Feb 6;42(4):819-827. doi: 10.1016/j.vaccine.2023.12.081. Epub 2024 Jan 12. Vaccine. 2024. PMID: 38218668 Free PMC article. Review.
-
Intestinal mucosal immunity is unimportant for polio eradication: the failure of oral polio vaccination.Infect Dis (Lond). 2024 Aug;56(8):669-677. doi: 10.1080/23744235.2024.2367742. Epub 2024 Jun 18. Infect Dis (Lond). 2024. PMID: 38889538 Review.
Cited by
-
Modeling Poliovirus Transmission and Responses in New York State.J Infect Dis. 2024 Apr 12;229(4):1097-1106. doi: 10.1093/infdis/jiad355. J Infect Dis. 2024. PMID: 37596838 Free PMC article.
-
Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences.Risk Anal. 2024 Feb;44(2):366-378. doi: 10.1111/risa.14158. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344934 Free PMC article. Review.
-
Outbreak response strategies with type 2-containing oral poliovirus vaccines.Vaccine. 2023 Apr 6;41 Suppl 1(Suppl 1):A142-A152. doi: 10.1016/j.vaccine.2022.10.060. Epub 2022 Nov 17. Vaccine. 2023. PMID: 36402659 Free PMC article.
-
Health economic analysis of vaccine options for the polio eradication endgame: 2022-2036.Expert Rev Vaccines. 2022 Nov;21(11):1667-1674. doi: 10.1080/14760584.2022.2128108. Epub 2022 Oct 5. Expert Rev Vaccines. 2022. PMID: 36154436 Free PMC article.
-
Modeling scenarios for ending poliovirus transmission in Pakistan and Afghanistan.Risk Anal. 2023 Apr;43(4):660-676. doi: 10.1111/risa.13983. Epub 2022 Jun 23. Risk Anal. 2023. PMID: 35739080 Free PMC article.
References
-
- Anis E, Kopel E, Singer S, Kaliner E, Moerman L, Moran-Gilad J, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveillance. 2013;18:pii=20586. - PubMed
-
- Shulman LM, Mendelson E, Anis E, Bassal R, Gdalevich M, Hindiyeh M, et al. Laboratory challenges in response to silent introduction and sustained transmission of wild type 1 poliovirus into Israel in 2013. J Infect Dis. 2014;210:S304–14. - PubMed
-
- World Health Organization. Poliovirus detected from environmental samples in Egypt. Geneva: WHO; 11 February 2013. Available from: http://wwwwhoint/csr/don/2013_02_11/en/ Accessed 27 October 2014. 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

