Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure
- PMID: 25700512
- PMCID: PMC4432913
- DOI: 10.1126/science.1256022
Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure
Abstract
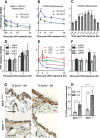
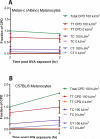
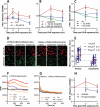
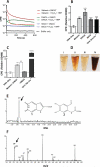
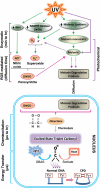
Mutations in sunlight-induced melanoma arise from cyclobutane pyrimidine dimers (CPDs), DNA photoproducts that are typically created picoseconds after an ultraviolet (UV) photon is absorbed at thymine or cytosine. We found that in melanocytes, CPDs are generated for >3 hours after exposure to UVA, a major component of the radiation in sunlight and in tanning beds. These "dark CPDs" constitute the majority of CPDs and include the cytosine-containing CPDs that initiate UV-signature C→T mutations. Dark CPDs arise when UV-induced reactive oxygen and nitrogen species combine to excite an electron in fragments of the pigment melanin. This creates a quantum triplet state that has the energy of a UV photon but induces CPDs by energy transfer to DNA in a radiation-independent manner. Melanin may thus be carcinogenic as well as protective against cancer. These findings also validate the long-standing suggestion that chemically generated excited electronic states are relevant to mammalian biology.
Copyright © 2015, American Association for the Advancement of Science.
Figures
Comment in
-
Biomolecules. The dark side of sunlight and melanoma.Science. 2015 Feb 20;347(6224):824. doi: 10.1126/science.aaa6578. Science. 2015. PMID: 25700500 No abstract available.
-
Dark CPDs and photocarcinogenesis: the party continues after the lights go out.Pigment Cell Melanoma Res. 2015 Jul;28(4):373-4. doi: 10.1111/pcmr.12381. Pigment Cell Melanoma Res. 2015. PMID: 25950490 No abstract available.
Similar articles
-
UV-induced Melanin Chemiexcitation: A New Mode of Melanoma Pathogenesis.Toxicol Pathol. 2016 Jun;44(4):552-4. doi: 10.1177/0192623316632072. Epub 2016 Mar 7. Toxicol Pathol. 2016. PMID: 26951162 Free PMC article.
-
[Ultraviolet A-induced DNA damage: role in skin cancer].Bull Acad Natl Med. 2014 Feb;198(2):273-95. Bull Acad Natl Med. 2014. PMID: 26263704 French.
-
Chemical excitation of electrons: A dark path to melanoma.DNA Repair (Amst). 2016 Aug;44:169-177. doi: 10.1016/j.dnarep.2016.05.023. Epub 2016 Jun 1. DNA Repair (Amst). 2016. PMID: 27262612 Free PMC article. Review.
-
Dark CPDs and photocarcinogenesis: the party continues after the lights go out.Pigment Cell Melanoma Res. 2015 Jul;28(4):373-4. doi: 10.1111/pcmr.12381. Pigment Cell Melanoma Res. 2015. PMID: 25950490 No abstract available.
-
Mechanistic considerations on the wavelength-dependent variations of UVR genotoxicity and mutagenesis in skin: the discrimination of UVA-signature from UV-signature mutation.Photochem Photobiol Sci. 2018 Dec 5;17(12):1861-1871. doi: 10.1039/c7pp00360a. Photochem Photobiol Sci. 2018. PMID: 29850669 Review.
Cited by
-
Cell-intrinsic melanin fails to protect melanocytes from ultraviolet-mutagenesis in the absence of epidermal melanin.Pigment Cell Melanoma Res. 2023 Jan;36(1):6-18. doi: 10.1111/pcmr.13070. Epub 2022 Oct 9. Pigment Cell Melanoma Res. 2023. PMID: 36148789 Free PMC article.
-
Role of Melanin Chemiexcitation in Melanoma Progression and Drug Resistance.Front Oncol. 2020 Aug 6;10:1305. doi: 10.3389/fonc.2020.01305. eCollection 2020. Front Oncol. 2020. PMID: 32850409 Free PMC article. Review.
-
MITF and UV responses in skin: From pigmentation to addiction.Pigment Cell Melanoma Res. 2019 Mar;32(2):224-236. doi: 10.1111/pcmr.12726. Epub 2018 Aug 3. Pigment Cell Melanoma Res. 2019. PMID: 30019545 Free PMC article. Review.
-
New insights and advances on pyomelanin production: from microbial synthesis to applications.J Ind Microbiol Biotechnol. 2022 Jul 30;49(4):kuac013. doi: 10.1093/jimb/kuac013. J Ind Microbiol Biotechnol. 2022. PMID: 35482661 Free PMC article. Review.
-
Redox-Related Proteins in Melanoma Progression.Antioxidants (Basel). 2022 Feb 22;11(3):438. doi: 10.3390/antiox11030438. Antioxidants (Basel). 2022. PMID: 35326089 Free PMC article.
References
-
- Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J. Photochem. Photobiol. B. 1991;9:135–160. - PubMed
-
- Williams PF, Olsen CM, Hayward NK, Whiteman DC. Melanocortin 1 receptor and risk of cutaneous melanoma: a meta-analysis and estimates of population burden. Int J Cancer. 2011;129:1730–1740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

