D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections
- PMID: 25699603
- PMCID: PMC4362967
- DOI: 10.1016/j.chembiol.2015.01.002
D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections
Erratum in
- Chem Biol. 2015 Sep 17;22(9):1280-2
Abstract
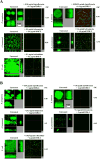
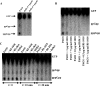
In many infections, bacteria form surface-associated communities known as biofilms that are substantially more resistant to antibiotics than their planktonic counterparts. Based on the design features of active antibiofilm peptides, we made a series of related 12-amino acid L-, D- and retro-inverso derivatives. Specific D-enantiomeric peptides were the most potent at inhibiting biofilm development and eradicating preformed biofilms of seven species of wild-type and multiply antibiotic-resistant Gram-negative pathogens. Moreover, these peptides showed strong synergy with conventional antibiotics, reducing the antibiotic concentrations required for complete biofilm inhibition by up to 64-fold. As shown previously for 1018, these D-amino acid peptides targeted the intracellular stringent response signal (p)ppGpp. The most potent peptides DJK-5 and DJK-6 protected invertebrates from lethal Pseudomonas aeruginosa infections and were considerably more active than a previously described L-amino acid peptide 1018. Thus, the protease-resistant peptides produced here were more effective both in vitro and in vivo.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii.Sci Rep. 2017 Jul 31;7(1):6953. doi: 10.1038/s41598-017-07440-0. Sci Rep. 2017. PMID: 28761101 Free PMC article.
-
Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm.Molecules. 2019 Dec 12;24(24):4560. doi: 10.3390/molecules24244560. Molecules. 2019. PMID: 31842508 Free PMC article.
-
Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides.Biomolecules. 2018 May 21;8(2):29. doi: 10.3390/biom8020029. Biomolecules. 2018. PMID: 29883434 Free PMC article.
-
Pseudomonas aeruginosa biofilm infections: from molecular biofilm biology to new treatment possibilities.APMIS Suppl. 2014 Dec;(138):1-51. doi: 10.1111/apm.12335. APMIS Suppl. 2014. PMID: 25399808 Review.
-
Antibiofilm Peptides: Potential as Broad-Spectrum Agents.J Bacteriol. 2016 Sep 9;198(19):2572-8. doi: 10.1128/JB.00017-16. Print 2016 Oct 1. J Bacteriol. 2016. PMID: 27068589 Free PMC article. Review.
Cited by
-
Microbial Biofilms in Pulmonary and Critical Care Diseases.Ann Am Thorac Soc. 2016 Sep;13(9):1615-23. doi: 10.1513/AnnalsATS.201603-194FR. Ann Am Thorac Soc. 2016. PMID: 27348071 Free PMC article. Review.
-
Harnessing biomolecules for bioinspired dental biomaterials.J Mater Chem B. 2020 Oct 14;8(38):8713-8747. doi: 10.1039/d0tb01456g. Epub 2020 Aug 4. J Mater Chem B. 2020. PMID: 32747882 Free PMC article. Review.
-
Current Promising Strategies against Antibiotic-Resistant Bacterial Infections.Antibiotics (Basel). 2022 Dec 30;12(1):67. doi: 10.3390/antibiotics12010067. Antibiotics (Basel). 2022. PMID: 36671268 Free PMC article. Review.
-
Pseudomonas aeruginosa Biofilm Dispersion by the Human Atrial Natriuretic Peptide.Adv Sci (Weinh). 2022 Mar;9(7):e2103262. doi: 10.1002/advs.202103262. Epub 2022 Jan 14. Adv Sci (Weinh). 2022. PMID: 35032112 Free PMC article.
-
Helicobacter pylori Biofilm Formation Is Differentially Affected by Common Culture Conditions, and Proteins Play a Central Role in the Biofilm Matrix.Appl Environ Microbiol. 2018 Jul 2;84(14):e00391-18. doi: 10.1128/AEM.00391-18. Print 2018 Jul 15. Appl Environ Microbiol. 2018. PMID: 29752266 Free PMC article.
References
-
- Aberg A, Shingler V, Balsalobre C. (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol Microbiol. 2006;60:1520–33. - PubMed
-
- Amer LS, Bishop BM, van Hoek ML. Antimicrobial and antibiofilm activity of cathelicidins and short, synthetic peptides against Francisella. Biochem Biophys Res Commun. 2010;396:246–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

