Gut/brain axis and the microbiota
- PMID: 25689247
- PMCID: PMC4362231
- DOI: 10.1172/JCI76304
Gut/brain axis and the microbiota
Abstract
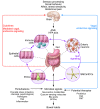
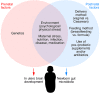
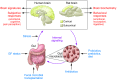
Tremendous progress has been made in characterizing the bidirectional interactions between the central nervous system, the enteric nervous system, and the gastrointestinal tract. A series of provocative preclinical studies have suggested a prominent role for the gut microbiota in these gut-brain interactions. Based on studies using rodents raised in a germ-free environment, the gut microbiota appears to influence the development of emotional behavior, stress- and pain-modulation systems, and brain neurotransmitter systems. Additionally, microbiota perturbations by probiotics and antibiotics exert modulatory effects on some of these measures in adult animals. Current evidence suggests that multiple mechanisms, including endocrine and neurocrine pathways, may be involved in gut microbiota-to-brain signaling and that the brain can in turn alter microbial composition and behavior via the autonomic nervous system. Limited information is available on how these findings may translate to healthy humans or to disease states involving the brain or the gut/brain axis. Future research needs to focus on confirming that the rodent findings are translatable to human physiology and to diseases such as irritable bowel syndrome, autism, anxiety, depression, and Parkinson's disease.
Figures
Similar articles
-
Gut Microbiota-brain Axis.Chin Med J (Engl). 2016 Oct 5;129(19):2373-80. doi: 10.4103/0366-6999.190667. Chin Med J (Engl). 2016. PMID: 27647198 Free PMC article. Review.
-
Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour.Acta Paediatr. 2014 Aug;103(8):812-9. doi: 10.1111/apa.12674. Epub 2014 May 17. Acta Paediatr. 2014. PMID: 24798884 Review.
-
Brain-gut-microbiota axis in Parkinson's disease.World J Gastroenterol. 2015 Oct 7;21(37):10609-20. doi: 10.3748/wjg.v21.i37.10609. World J Gastroenterol. 2015. PMID: 26457021 Free PMC article. Review.
-
Mind-altering with the gut: Modulation of the gut-brain axis with probiotics.J Microbiol. 2018 Mar;56(3):172-182. doi: 10.1007/s12275-018-8032-4. Epub 2018 Feb 28. J Microbiol. 2018. PMID: 29492874 Review.
-
The gut microbiome and the brain.J Med Food. 2014 Dec;17(12):1261-72. doi: 10.1089/jmf.2014.7000. J Med Food. 2014. PMID: 25402818 Free PMC article. Review.
Cited by
-
The gut connectome: making sense of what you eat.J Clin Invest. 2015 Mar 2;125(3):888-90. doi: 10.1172/JCI81121. Epub 2015 Mar 2. J Clin Invest. 2015. PMID: 25729849 Free PMC article.
-
Causal associations between gut microbiota and regional cortical structure: a Mendelian randomization study.Front Neurosci. 2023 Dec 22;17:1296145. doi: 10.3389/fnins.2023.1296145. eCollection 2023. Front Neurosci. 2023. PMID: 38196849 Free PMC article.
-
Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults.Nutrients. 2020 Jul 21;12(7):2164. doi: 10.3390/nu12072164. Nutrients. 2020. PMID: 32708278 Free PMC article.
-
Altered physiology of gastrointestinal vagal afferents following neurotrauma.Neural Regen Res. 2021 Feb;16(2):254-263. doi: 10.4103/1673-5374.290883. Neural Regen Res. 2021. PMID: 32859772 Free PMC article. Review.
-
Association of low-grade inflammation caused by gut microbiota disturbances with osteoarthritis: A systematic review.Front Vet Sci. 2022 Sep 12;9:938629. doi: 10.3389/fvets.2022.938629. eCollection 2022. Front Vet Sci. 2022. PMID: 36172610 Free PMC article.
References
-
- de Vos WM, de Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev. 2012;70(suppl 1):S45–S56. - PubMed

