A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases
- PMID: 25686105
- PMCID: PMC4392179
- DOI: 10.1038/nm.3806
A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases
Abstract
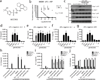
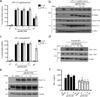
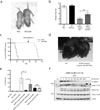
The NOD-like receptor (NLR) family, pyrin domain-containing protein 3 (NLRP3) inflammasome is a component of the inflammatory process, and its aberrant activation is pathogenic in inherited disorders such as cryopyrin-associated periodic syndrome (CAPS) and complex diseases such as multiple sclerosis, type 2 diabetes, Alzheimer's disease and atherosclerosis. We describe the development of MCC950, a potent, selective, small-molecule inhibitor of NLRP3. MCC950 blocked canonical and noncanonical NLRP3 activation at nanomolar concentrations. MCC950 specifically inhibited activation of NLRP3 but not the AIM2, NLRC4 or NLRP1 inflammasomes. MCC950 reduced interleukin-1β (IL-1β) production in vivo and attenuated the severity of experimental autoimmune encephalomyelitis (EAE), a disease model of multiple sclerosis. Furthermore, MCC950 treatment rescued neonatal lethality in a mouse model of CAPS and was active in ex vivo samples from individuals with Muckle-Wells syndrome. MCC950 is thus a potential therapeutic for NLRP3-associated syndromes, including autoinflammatory and autoimmune diseases, and a tool for further study of the NLRP3 inflammasome in human health and disease.
Figures



Comment in
-
Inflammation. Potent small molecule extinguishes the NLRP3 inflammasome.Nat Rev Rheumatol. 2015 Apr;11(4):198. doi: 10.1038/nrrheum.2015.23. Epub 2015 Mar 3. Nat Rev Rheumatol. 2015. PMID: 25734976 No abstract available.
-
Inflammasome: starving inflammation.Nat Rev Immunol. 2015 Apr;15(4):199. doi: 10.1038/nri3832. Epub 2015 Mar 6. Nat Rev Immunol. 2015. PMID: 25743221 No abstract available.
-
Inflammasome inhibition: putting out the fire.Cell Metab. 2015 Apr 7;21(4):513-4. doi: 10.1016/j.cmet.2015.03.012. Cell Metab. 2015. PMID: 25863243
-
The Nlrp3 inflammasome admits defeat.Trends Immunol. 2015 Jun;36(6):323-4. doi: 10.1016/j.it.2015.05.001. Epub 2015 May 16. Trends Immunol. 2015. PMID: 25991463
Similar articles
-
Inflammation. Potent small molecule extinguishes the NLRP3 inflammasome.Nat Rev Rheumatol. 2015 Apr;11(4):198. doi: 10.1038/nrrheum.2015.23. Epub 2015 Mar 3. Nat Rev Rheumatol. 2015. PMID: 25734976 No abstract available.
-
MCC950 in the treatment of NLRP3-mediated inflammatory diseases: Latest evidence and therapeutic outcomes.Int Immunopharmacol. 2022 May;106:108595. doi: 10.1016/j.intimp.2022.108595. Epub 2022 Feb 3. Int Immunopharmacol. 2022. PMID: 35124417 Review.
-
The Nlrp3 inflammasome admits defeat.Trends Immunol. 2015 Jun;36(6):323-4. doi: 10.1016/j.it.2015.05.001. Epub 2015 May 16. Trends Immunol. 2015. PMID: 25991463
-
MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition.PLoS Biol. 2019 Sep 16;17(9):e3000354. doi: 10.1371/journal.pbio.3000354. eCollection 2019 Sep. PLoS Biol. 2019. PMID: 31525186 Free PMC article.
-
Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome.Eur J Pharmacol. 2022 Aug 5;928:175091. doi: 10.1016/j.ejphar.2022.175091. Epub 2022 Jun 14. Eur J Pharmacol. 2022. PMID: 35714692 Review.
Cited by
-
Targeting the inflammasome in Parkinson's disease.Front Aging Neurosci. 2022 Oct 12;14:957705. doi: 10.3389/fnagi.2022.957705. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36313019 Free PMC article.
-
Inflammation. Potent small molecule extinguishes the NLRP3 inflammasome.Nat Rev Rheumatol. 2015 Apr;11(4):198. doi: 10.1038/nrrheum.2015.23. Epub 2015 Mar 3. Nat Rev Rheumatol. 2015. PMID: 25734976 No abstract available.
-
IP3R2-mediated Ca2+ release promotes LPS-induced cardiomyocyte pyroptosis via the activation of NLRP3/Caspase-1/GSDMD pathway.Cell Death Discov. 2024 Feb 20;10(1):91. doi: 10.1038/s41420-024-01840-8. Cell Death Discov. 2024. PMID: 38378646 Free PMC article.
-
NLRP3 inflammasome activation mechanism and its role in autoimmune liver disease.Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(11):1577-1586. doi: 10.3724/abbs.2022137. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36148948 Free PMC article. Review.
-
Selective inhibition of the K+ efflux sensitive NLRP3 pathway by Cl- channel modulation.Chem Sci. 2020 Oct 12;11(43):11720-11728. doi: 10.1039/d0sc03828h. Chem Sci. 2020. PMID: 34094411 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

