How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions
- PMID: 25683596
- PMCID: PMC4440321
- DOI: 10.1016/j.jmb.2015.02.004
How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions
Abstract
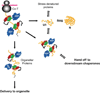
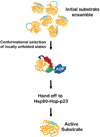
Hsp70 molecular chaperones are implicated in a wide variety of cellular processes, including protein biogenesis, protection of the proteome from stress, recovery of proteins from aggregates, facilitation of protein translocation across membranes, and more specialized roles such as disassembly of particular protein complexes. It is a fascinating question to ask how the mechanism of these deceptively simple molecular machines is matched to their roles in these wide-ranging processes. The key is a combination of the nature of the recognition and binding of Hsp70 substrates and the impact of Hsp70 action on their substrates. In many cases, the binding, which relies on interaction with an extended, accessible short hydrophobic sequence, favors more unfolded states of client proteins. The ATP-mediated dissociation of the substrate thus releases it in a relatively less folded state for downstream folding, membrane translocation, or hand-off to another chaperone. There are cases, such as regulation of the heat shock response or disassembly of clathrin coats, however, where binding of a short hydrophobic sequence selects conformational states of clients to favor their productive participation in a subsequent step. This Perspective discusses current understanding of how Hsp70 molecular chaperones recognize and act on their substrates and the relationships between these fundamental processes and the functional roles played by these molecular machines.
Keywords: Hsp70 molecular chaperone; chaperone substrates; complex disassembly; disaggregation; protein folding.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Chaperone machines for protein folding, unfolding and disaggregation.Nat Rev Mol Cell Biol. 2013 Oct;14(10):630-42. doi: 10.1038/nrm3658. Epub 2013 Sep 12. Nat Rev Mol Cell Biol. 2013. PMID: 24026055 Free PMC article. Review.
-
Alternative modes of client binding enable functional plasticity of Hsp70.Nature. 2016 Nov 17;539(7629):448-451. doi: 10.1038/nature20137. Epub 2016 Oct 26. Nature. 2016. PMID: 27783598
-
Hsp70 molecular chaperones: multifunctional allosteric holding and unfolding machines.Biochem J. 2019 Jun 14;476(11):1653-1677. doi: 10.1042/BCJ20170380. Biochem J. 2019. PMID: 31201219 Free PMC article. Review.
-
Experimental Milestones in the Discovery of Molecular Chaperones as Polypeptide Unfolding Enzymes.Annu Rev Biochem. 2016 Jun 2;85:715-42. doi: 10.1146/annurev-biochem-060815-014124. Epub 2016 Mar 31. Annu Rev Biochem. 2016. PMID: 27050154 Review.
-
More than folding: localized functions of cytosolic chaperones.Trends Biochem Sci. 2003 Oct;28(10):541-7. doi: 10.1016/j.tibs.2003.08.009. Trends Biochem Sci. 2003. PMID: 14559183 Review.
Cited by
-
UNC-45 assisted myosin folding depends on a conserved FX3HY motif implicated in Freeman Sheldon Syndrome.Nat Commun. 2024 Jul 25;15(1):6272. doi: 10.1038/s41467-024-50442-6. Nat Commun. 2024. PMID: 39054317 Free PMC article.
-
Molecular chaperones: providing a safe place to weather a midlife protein-folding crisis.Nat Struct Mol Biol. 2016 Jul 6;23(7):621-3. doi: 10.1038/nsmb.3255. Nat Struct Mol Biol. 2016. PMID: 27384188 No abstract available.
-
head-bent resistant Hsc70 variants show reduced Hsp40 affinity and altered protein folding activity.Sci Rep. 2019 Aug 16;9(1):11955. doi: 10.1038/s41598-019-48109-0. Sci Rep. 2019. PMID: 31420580 Free PMC article.
-
Distribution and solvent exposure of Hsp70 chaperone binding sites across the Escherichia coli proteome.Proteins. 2023 May;91(5):665-678. doi: 10.1002/prot.26456. Epub 2023 Jan 1. Proteins. 2023. PMID: 36539330 Free PMC article.
-
Binding with heat shock cognate protein HSC70 fine-tunes the Golgi association of the small GTPase ARL5B.J Biol Chem. 2021 Dec;297(6):101422. doi: 10.1016/j.jbc.2021.101422. Epub 2021 Nov 16. J Biol Chem. 2021. PMID: 34798070 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

