Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis
- PMID: 25681733
- PMCID: PMC4380846
- DOI: 10.1016/j.ajpath.2014.12.005
Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis
Abstract
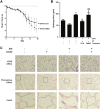
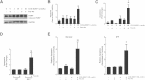
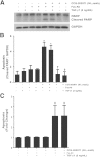
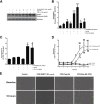
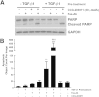
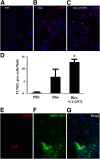
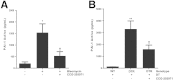
Myofibroblasts are crucial to the pathogenesis of tissue fibrosis. Their formation of stress fibers results in the release of myocardin-related transcription factor (MRTF), a transcriptional coactivator of serum response factor (SRF). MRTF-A (Mkl1)-deficient mice are protected from lung fibrosis. We hypothesized that the SRF/MRTF pathway inhibitor CCG-203971 would modulate myofibroblast function in vitro and limit lung fibrosis in vivo. Normal and idiopathic pulmonary fibrosis lung fibroblasts were treated with/without CCG-203971 (N-[4-chlorophenyl]-1-[3-(2-furanyl)benzoyl]-3-piperidine carboxamide) and/or Fas-activating antibody in the presence/absence of transforming growth factor (TGF)-β1, and apoptosis was assessed. In vivo studies examined the effect of therapeutically administered CCG-203971 on lung fibrosis in two distinct murine models of fibrosis induced by bleomycin or targeted type II alveolar epithelial injury. In vitro, CCG-203971 prevented nuclear localization of MRTF-A; increased the apoptotic susceptibility of normal and idiopathic pulmonary fibrosis fibroblasts; blocked TGF-β1-induced myofibroblast differentiation; and inhibited TGF-β1-induced expression of fibronectin, X-linked inhibitor of apoptosis, and plasminogen activator inhibitor-1. TGF-β1 did not protect fibroblasts or myofibroblasts from apoptosis in the presence of CCG-203971. In vivo, CCG-203971 significantly reduced lung collagen content in both murine models while decreasing alveolar plasminogen activator inhibitor-1 and promoting myofibroblast apoptosis. These data support a central role of the SRF/MRTF pathway in the pathobiology of lung fibrosis and suggest that its inhibition can help resolve lung fibrosis by promoting fibroblast apoptosis.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
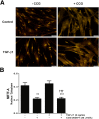
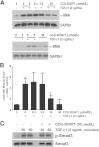
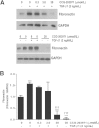
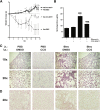

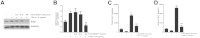
Comment in
-
ROCK and Rho: Promising therapeutic targets to ameliorate pulmonary fibrosis.Am J Pathol. 2015 Apr;185(4):909-12. doi: 10.1016/j.ajpath.2015.01.005. Epub 2015 Feb 14. Am J Pathol. 2015. PMID: 25687558 Free PMC article.
Similar articles
-
Umbilical cord/placenta-derived mesenchymal stem cells inhibit fibrogenic activation in human intestinal myofibroblasts via inhibition of myocardin-related transcription factor A.Stem Cell Res Ther. 2019 Sep 23;10(1):291. doi: 10.1186/s13287-019-1385-8. Stem Cell Res Ther. 2019. PMID: 31547873 Free PMC article.
-
Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β.Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L656-66. doi: 10.1152/ajplung.00166.2011. Epub 2011 Aug 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21856814 Free PMC article.
-
Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts.Inflamm Bowel Dis. 2014 Jan;20(1):154-65. doi: 10.1097/01.MIB.0000437615.98881.31. Inflamm Bowel Dis. 2014. PMID: 24280883 Free PMC article.
-
The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation.J Cardiovasc Transl Res. 2012 Dec;5(6):794-804. doi: 10.1007/s12265-012-9397-0. Epub 2012 Aug 17. J Cardiovasc Transl Res. 2012. PMID: 22898751 Review.
-
Transcriptional control of cardiac fibroblast plasticity.J Mol Cell Cardiol. 2016 Feb;91:52-60. doi: 10.1016/j.yjmcc.2015.12.016. Epub 2015 Dec 22. J Mol Cell Cardiol. 2016. PMID: 26721596 Free PMC article. Review.
Cited by
-
Moesin, an Ezrin/Radixin/Moesin Family Member, Regulates Hepatic Fibrosis.Hepatology. 2020 Sep;72(3):1073-1084. doi: 10.1002/hep.31078. Epub 2020 Jul 9. Hepatology. 2020. PMID: 31860744 Free PMC article.
-
Resident Fibroblast MKL1 Is Sufficient to Drive Pro-fibrogenic Response in Mice.Front Cell Dev Biol. 2022 Feb 1;9:812748. doi: 10.3389/fcell.2021.812748. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35178401 Free PMC article.
-
Cyclosporine A inhibits MRTF-SRF signaling through Na+/K+ ATPase inhibition and actin remodeling.FASEB Bioadv. 2019 Aug 24;1(9):561-578. doi: 10.1096/fba.2019-00027. eCollection 2019 Sep. FASEB Bioadv. 2019. PMID: 32123851 Free PMC article.
-
Current state of signaling pathways associated with the pathogenesis of idiopathic pulmonary fibrosis.Respir Res. 2024 Jun 17;25(1):245. doi: 10.1186/s12931-024-02878-z. Respir Res. 2024. PMID: 38886743 Free PMC article. Review.
-
Pharmacokinetic optimitzation of CCG-203971: Novel inhibitors of the Rho/MRTF/SRF transcriptional pathway as potential antifibrotic therapeutics for systemic scleroderma.Bioorg Med Chem Lett. 2017 Apr 15;27(8):1744-1749. doi: 10.1016/j.bmcl.2017.02.070. Epub 2017 Mar 10. Bioorg Med Chem Lett. 2017. PMID: 28285914 Free PMC article.
References
-
- Raghu G., Chen S.Y., Yeh W.S., Maroni B., Li Q., Lee Y.C., Collard H.R. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. [erratum in: Lancet Respir Med 2014;2:e12] - PubMed
-
- Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.F., Flaherty K.R., Lasky J.A., Lynch D.A., Ryu J.H., Swigris J.J., Wells A.U., Ancochea J., Bouros D., Carvalho C., Costabel U., Ebina M., Hansell D.M., Johkoh T., Kim D.S., King T.E., Jr., Kondoh Y., Myers J., Müller N.L., Nicholson A.G., Richeldi L., Selman M., Dudden R.F., Griss B.S., Protzko S.L., Schünemann H.J. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis: An official Ats/Ers/Jrs/Alat statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- Follonier L., Schaub S., Meister J.J., Hinz B. Myofibroblast communication is controlled by intercellular mechanical coupling. J Cell Sci. 2008;121:3305–3316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1S10RR27525/RR/NCRR NIH HHS/United States
- R01 HL105489/HL/NHLBI NIH HHS/United States
- HL108904/HL/NHLBI NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- HL078871/HL/NHLBI NIH HHS/United States
- R01 AR066049/AR/NIAMS NIH HHS/United States
- R01AR066049/AR/NIAMS NIH HHS/United States
- R01 HL124076/HL/NHLBI NIH HHS/United States
- R01 HL078871/HL/NHLBI NIH HHS/United States
- R01 HL108904/HL/NHLBI NIH HHS/United States
- S10 RR027525/RR/NCRR NIH HHS/United States
- HL105489/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

