Visualizing the functional architecture of the endocytic machinery
- PMID: 25675087
- PMCID: PMC4357291
- DOI: 10.7554/eLife.04535
Visualizing the functional architecture of the endocytic machinery
Abstract
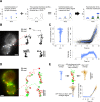
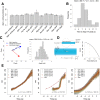
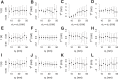
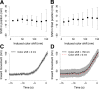
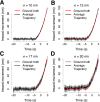
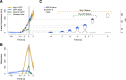
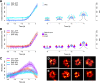
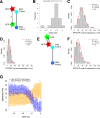
Clathrin-mediated endocytosis is an essential process that forms vesicles from the plasma membrane. Although most of the protein components of the endocytic protein machinery have been thoroughly characterized, their organization at the endocytic site is poorly understood. We developed a fluorescence microscopy method to track the average positions of yeast endocytic proteins in relation to each other with a time precision below 1 s and with a spatial precision of ~10 nm. With these data, integrated with shapes of endocytic membrane intermediates and with superresolution imaging, we could visualize the dynamic architecture of the endocytic machinery. We showed how different coat proteins are distributed within the coat structure and how the assembly dynamics of N-BAR proteins relate to membrane shape changes. Moreover, we found that the region of actin polymerization is located at the base of the endocytic invagination, with the growing ends of filaments pointing toward the plasma membrane.
Keywords: S. cerevisiae; actin; biophysics; cell biology; endocytosis; live-cell imaging; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





Similar articles
-
Precise tracking of the dynamics of multiple proteins in endocytic events.Methods Cell Biol. 2017;139:51-68. doi: 10.1016/bs.mcb.2016.11.002. Epub 2016 Dec 29. Methods Cell Biol. 2017. PMID: 28215339
-
A modular design for the clathrin- and actin-mediated endocytosis machinery.Cell. 2005 Oct 21;123(2):305-20. doi: 10.1016/j.cell.2005.09.024. Cell. 2005. PMID: 16239147
-
Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography.Cell. 2012 Aug 3;150(3):508-20. doi: 10.1016/j.cell.2012.05.046. Cell. 2012. PMID: 22863005
-
Actin assembly and endocytosis: from yeast to mammals.Annu Rev Cell Dev Biol. 2003;19:287-332. doi: 10.1146/annurev.cellbio.19.111401.093127. Annu Rev Cell Dev Biol. 2003. PMID: 14570572 Review.
-
Seeing is believing: imaging actin dynamics at single sites of endocytosis.Trends Cell Biol. 2004 Jul;14(7):352-8. doi: 10.1016/j.tcb.2004.05.008. Trends Cell Biol. 2004. PMID: 15246428 Review.
Cited by
-
Spatio-temporal regulation of endocytic protein assembly by SH3 domains in yeast.Mol Biol Cell. 2023 Mar 1;34(3):ar19. doi: 10.1091/mbc.E22-09-0406. Epub 2023 Jan 25. Mol Biol Cell. 2023. PMID: 36696224 Free PMC article.
-
Condensation of Ede1 promotes the initiation of endocytosis.Elife. 2022 Apr 12;11:e72865. doi: 10.7554/eLife.72865. Elife. 2022. PMID: 35412456 Free PMC article.
-
Mapping molecular complexes with super-resolution microscopy and single-particle analysis.Open Biol. 2022 Jul;12(7):220079. doi: 10.1098/rsob.220079. Epub 2022 Jul 27. Open Biol. 2022. PMID: 35892200 Free PMC article. Review.
-
Design principles for robust vesiculation in clathrin-mediated endocytosis.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1118-E1127. doi: 10.1073/pnas.1617705114. Epub 2017 Jan 26. Proc Natl Acad Sci U S A. 2017. PMID: 28126722 Free PMC article.
-
Vesiculation pathways in clathrin-mediated endocytosis.bioRxiv [Preprint]. 2024 Sep 23:2024.08.13.607731. doi: 10.1101/2024.08.13.607731. bioRxiv. 2024. PMID: 39185216 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

