Elevated cytokines, thrombin and PAI-1 in severe HCPS patients due to Sin Nombre virus
- PMID: 25674766
- PMCID: PMC4353904
- DOI: 10.3390/v7020559
Elevated cytokines, thrombin and PAI-1 in severe HCPS patients due to Sin Nombre virus
Abstract
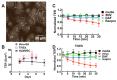
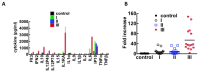
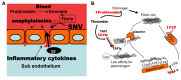
Sin Nombre Hantavirus (SNV, Bunyaviridae Hantavirus) is a Category A pathogen that causes Hantavirus Cardiopulmonary Syndrome (HCPS) with case fatality ratios generally ranging from 30% to 50%. HCPS is characterized by vascular leakage due to dysregulation of the endothelial barrier function. The loss of vascular integrity results in non-cardiogenic pulmonary edema, shock, multi-organ failure and death. Using Electric Cell-substrate Impedance Sensing (ECIS) measurements, we found that plasma samples drawn from University of New Mexico Hospital patients with serologically-confirmed HCPS, induce loss of cell-cell adhesion in confluent epithelial and endothelial cell monolayers grown in ECIS cultureware. We show that the loss of cell-cell adhesion is sensitive to both thrombin and plasmin inhibitors in mild cases, and to thrombin only inhibition in severe cases, suggesting an increasing prothrombotic state with disease severity. A proteomic profile (2D gel electrophoresis and mass spectrometry) of HCPS plasma samples in our cohort revealed robust antifibrinolytic activity among terminal case patients. The prothrombotic activity is highlighted by acute ≥30 to >100 fold increases in active plasminogen activator inhibitor (PAI-1) which, preceded death of the subjects within 48 h. Taken together, this suggests that PAI-1 might be a response to the severe pathology as it is expected to reduce plasmin activity and possibly thrombin activity in the terminal patients.
Figures

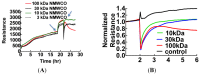



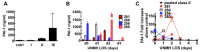
Similar articles
-
Upregulation of P2Y2R, Active uPA, and PAI-1 Are Essential Components of Hantavirus Cardiopulmonary Syndrome.Front Cell Infect Microbiol. 2018 May 23;8:169. doi: 10.3389/fcimb.2018.00169. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29930915 Free PMC article.
-
Longitudinal Assessment of Cytokine Expression and Plasminogen Activation in Hantavirus Cardiopulmonary Syndrome Reveals Immune Regulatory Dysfunction in End-Stage Disease.Viruses. 2021 Aug 12;13(8):1597. doi: 10.3390/v13081597. Viruses. 2021. PMID: 34452463 Free PMC article.
-
Neutralizing antibodies and Sin Nombre virus RNA after recovery from hantavirus cardiopulmonary syndrome.Emerg Infect Dis. 2004 Mar;10(3):478-82. doi: 10.3201/eid1003.020821. Emerg Infect Dis. 2004. PMID: 15109416 Free PMC article.
-
Hantavirus Cardiopulmonary Syndrome in Canada.Emerg Infect Dis. 2020 Dec;26(12):3020-3024. doi: 10.3201/eid2612.202808. Emerg Infect Dis. 2020. PMID: 33219792 Free PMC article. Review.
-
The role of endothelial activation in dengue hemorrhagic fever and hantavirus pulmonary syndrome.Virulence. 2013 Aug 15;4(6):525-36. doi: 10.4161/viru.25569. Epub 2013 Jul 10. Virulence. 2013. PMID: 23841977 Free PMC article. Review.
Cited by
-
Upregulation of P2Y2R, Active uPA, and PAI-1 Are Essential Components of Hantavirus Cardiopulmonary Syndrome.Front Cell Infect Microbiol. 2018 May 23;8:169. doi: 10.3389/fcimb.2018.00169. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29930915 Free PMC article.
-
Differential Regulation of PAI-1 in Hantavirus Cardiopulmonary Syndrome and Hemorrhagic Fever With Renal Syndrome.Open Forum Infect Dis. 2018 Jan 17;5(2):ofy021. doi: 10.1093/ofid/ofy021. eCollection 2018 Feb. Open Forum Infect Dis. 2018. PMID: 29450213 Free PMC article.
-
Exploration of the effects of goose TCs on GCs at different follicular stages using a co-culture model.Biosci Rep. 2020 Aug 28;40(8):BSR20200445. doi: 10.1042/BSR20200445. Biosci Rep. 2020. PMID: 32706022 Free PMC article.
-
Small-Volume Flow Cytometry-Based Multiplex Analysis of the Activity of Small GTPases.Methods Mol Biol. 2018;1821:177-195. doi: 10.1007/978-1-4939-8612-5_13. Methods Mol Biol. 2018. PMID: 30062413 Free PMC article.
-
Hantavirus Infection Suppresses Thrombospondin-1 Expression in Cultured Endothelial Cells in a Strain-Specific Manner.Front Microbiol. 2016 Jul 19;7:1077. doi: 10.3389/fmicb.2016.01077. eCollection 2016. Front Microbiol. 2016. PMID: 27486439 Free PMC article.
References
-
- Hjelle B. Epidemiology and diagnosis of hantavirus infections. In: Rose B.D., editor. UpToDate. Wolters Kluwer; Wellesley, MA, USA: 2014.
-
- Botten J., Mirowsky K., Kusewitt D., Ye C., Gottlieb K., Prescott J., Hjelle B. Persistent sin nombre virus infection in the deer mouse ( peromyscus maniculatus ) model: Sites of replication and strand specific expression. J. Virol. 2003;77:1540–1550. doi: 10.1128/JVI.77.2.1540-1550.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

