Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium
- PMID: 25673836
- PMCID: PMC4323526
- DOI: 10.1523/JNEUROSCI.3279-14.2015
Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium
Abstract
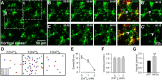
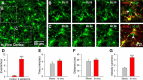
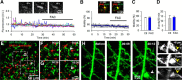
Extracellular calcium concentrations in the brain fluctuate during neuronal activities and may affect the behavior of brain cells. Microglia are highly dynamic immune cells of the brain. However, the effects of extracellular calcium concentrations on microglial dynamics have not been investigated. Here, we addressed this question in mouse brain slices and in vivo using two-photon microscopy. We serendipitously found that extracellular calcium reduction induced microglial processes to converge at distinct sites, a phenomenon we termed microglial process convergence (MPCs). Our studies revealed that MPCs target neuronal dendrites independent of neuronal action potential firing and is mediated by ATP release and microglial P2Y12 receptors. These results indicate that microglia monitor and interact with neurons during conditions of cerebral calcium reduction in the normal and diseased brain.
Keywords: P2Y12 receptor; calcium reduction; microglia; microglial process convergence; neuronal dendrites.
Copyright © 2015 the authors 0270-6474/15/352417-06$15.00/0.
Figures

Similar articles
-
Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus.J Neurosci. 2014 Aug 6;34(32):10528-40. doi: 10.1523/JNEUROSCI.0416-14.2014. J Neurosci. 2014. PMID: 25100587 Free PMC article.
-
Distinct P2Y Receptors Mediate Extension and Retraction of Microglial Processes in Epileptic and Peritumoral Human Tissue.J Neurosci. 2020 Feb 12;40(7):1373-1388. doi: 10.1523/JNEUROSCI.0218-19.2019. Epub 2020 Jan 2. J Neurosci. 2020. PMID: 31896671 Free PMC article.
-
Microglial P2Y12 deficiency/inhibition protects against brain ischemia.PLoS One. 2013 Aug 5;8(8):e70927. doi: 10.1371/journal.pone.0070927. Print 2013. PLoS One. 2013. PMID: 23940669 Free PMC article.
-
Dynamic motility of microglia: purinergic modulation of microglial movement in the normal and pathological brain.Glia. 2011 Dec;59(12):1793-9. doi: 10.1002/glia.21238. Epub 2011 Sep 7. Glia. 2011. PMID: 21901756 Review.
-
A decade of diverse microglial-neuronal physical interactions in the brain (2008-2018).Neurosci Lett. 2019 Apr 17;698:33-38. doi: 10.1016/j.neulet.2019.01.001. Epub 2019 Jan 6. Neurosci Lett. 2019. PMID: 30625349 Free PMC article. Review.
Cited by
-
FGF-1 Triggers Pannexin-1 Hemichannel Opening in Spinal Astrocytes of Rodents and Promotes Inflammatory Responses in Acute Spinal Cord Slices.J Neurosci. 2016 Apr 27;36(17):4785-801. doi: 10.1523/JNEUROSCI.4195-15.2016. J Neurosci. 2016. PMID: 27122036 Free PMC article.
-
Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke.Nat Commun. 2016 May 3;7:11499. doi: 10.1038/ncomms11499. Nat Commun. 2016. PMID: 27139776 Free PMC article.
-
Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury.Nat Commun. 2016 Jun 28;7:12029. doi: 10.1038/ncomms12029. Nat Commun. 2016. PMID: 27349690 Free PMC article.
-
Neuronal nuclear calcium signaling suppression of microglial reactivity is mediated by osteoprotegerin after traumatic brain injury.J Neuroinflammation. 2022 Nov 19;19(1):279. doi: 10.1186/s12974-022-02634-4. J Neuroinflammation. 2022. PMID: 36403069 Free PMC article.
-
The Emerging Role of Microglia in Neuromyelitis Optica.Front Immunol. 2021 Feb 19;12:616301. doi: 10.3389/fimmu.2021.616301. eCollection 2021. Front Immunol. 2021. PMID: 33679755 Free PMC article. Review.
References
-
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
