Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction
- PMID: 25660055
- PMCID: PMC4458286
- DOI: 10.1093/eurheartj/ehv007
Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction
Abstract
Aims: Neutrophil extracellular traps (NETs) are chromatin filaments released by activated polymorphonuclear neutrophils (PMNs) and decorated with granule proteins with various properties. Several lines of evidence implicate NETs in thrombosis. The functional significance and the in vivo relevance of NETs during atherothrombosis in humans have not been addressed until now.
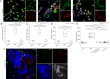
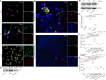
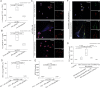
Methods and results: Selective sampling of thrombotic material and surrounding blood from the infarct-related coronary artery (IRA) and the non-IRA was performed during primary percutaneous revascularization in 18 patients with ST-segment elevation acute myocardial infarction (STEMI). Thrombi isolated from IRA contained PMNs and NETs decorated with tissue factor (TF). Although TF was expressed intracellularly in circulating PMNs of STEMI patients, active TF was specifically exposed by NETs obtained from the site of plaque rupture. Treatment of NET structures with DNase I abolished TF functionality measurement. In vitro treatment of control PMNs with plasma obtained from IRA and non-IRA was further shown to induce intracellular up-regulation of TF but not NET formation. A second step consisting of the interaction between PMNs and thrombin-activated platelets was required for NET generation and subsequent TF exposure.
Conclusion: The interaction of thrombin-activated platelets with PMNs at the site of plaque rupture during acute STEMI results in local NET formation and delivery of active TF. The notion that NETs represent a mechanism by which PMNs release thrombogenic signals during atherothrombosis may offer novel therapeutic targets.
Keywords: Inflammation; Myocardial infarction; Neutrophil extracellular traps; Thrombosis; Tissue factor.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




Comment in
-
Neutrophil extracellular traps: a new source of tissue factor in atherothrombosis.Eur Heart J. 2015 Jun 7;36(22):1364-6. doi: 10.1093/eurheartj/ehv105. Epub 2015 Apr 5. Eur Heart J. 2015. PMID: 25845929 No abstract available.
Similar articles
-
Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps.Int J Mol Sci. 2020 May 21;21(10):3625. doi: 10.3390/ijms21103625. Int J Mol Sci. 2020. PMID: 32455533 Free PMC article.
-
Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease.Ann Rheum Dis. 2014 Oct;73(10):1854-63. doi: 10.1136/annrheumdis-2013-203430. Epub 2013 Jul 19. Ann Rheum Dis. 2014. PMID: 23873874
-
Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury.Front Cell Infect Microbiol. 2021 Jul 14;11:677902. doi: 10.3389/fcimb.2021.677902. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34336711 Free PMC article.
-
Neutrophil Extracellular Traps in Arterial and Venous Thrombosis.Semin Thromb Hemost. 2019 Feb;45(1):86-93. doi: 10.1055/s-0038-1677040. Epub 2019 Jan 11. Semin Thromb Hemost. 2019. PMID: 30634198 Review.
-
Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis.Circ Res. 2017 Feb 17;120(4):736-743. doi: 10.1161/CIRCRESAHA.116.309692. Circ Res. 2017. PMID: 28209798 Review.
Cited by
-
Persistent neutrophilia is a marker for an increased risk of venous thrombosis.J Thromb Thrombolysis. 2016 Nov;42(4):545-51. doi: 10.1007/s11239-016-1398-4. J Thromb Thrombolysis. 2016. PMID: 27383828
-
Association of immunologic findings of atheromatous plaques with subsequent cardiovascular events in patients with peripheral artery disease.Sci Rep. 2024 Jan 3;14(1):469. doi: 10.1038/s41598-023-50751-8. Sci Rep. 2024. PMID: 38172197 Free PMC article.
-
Neutrophil-Platelet Interactions as Novel Treatment Targets in Cardiovascular Disease.Front Cardiovasc Med. 2022 Jan 31;8:824112. doi: 10.3389/fcvm.2021.824112. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35174225 Free PMC article. Review.
-
Potential Role of Neutrophil Extracellular Traps in Cardio-Oncology.Int J Mol Sci. 2022 Mar 25;23(7):3573. doi: 10.3390/ijms23073573. Int J Mol Sci. 2022. PMID: 35408933 Free PMC article. Review.
-
The Expanding Role of Extracellular Traps in Inflammation and Autoimmunity: The New Players in Casting Dark Webs.Int J Mol Sci. 2022 Mar 30;23(7):3793. doi: 10.3390/ijms23073793. Int J Mol Sci. 2022. PMID: 35409152 Free PMC article. Review.
References
-
- Rapaport SI, Rao LV. The tissue factor pathway: how it has become a “prima ballerina”. Thromb Haemost 1995;74:7–17. - PubMed
-
- Darbousset R, Thomas GM, Mezouar S, Frere C, Bonier R, Mackman N, Renne T, Dignat-George F, Dubois C, Panicot-Dubois L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012;120:2133–2143. - PubMed
-
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007;27:1687–1693. - PubMed
-
- von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012;209:819–835. - PMC - PubMed
-
- Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol 2006;177:4794–4802. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

