Roflumilast improves corticosteroid resistance COPD bronchial epithelial cells stimulated with toll like receptor 3 agonist
- PMID: 25652132
- PMCID: PMC4335416
- DOI: 10.1186/s12931-015-0179-5
Roflumilast improves corticosteroid resistance COPD bronchial epithelial cells stimulated with toll like receptor 3 agonist
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterised by chronic pulmonary inflammation punctuated by periods of viral exacerbations. Recent evidence suggests that the combination of roflumilast with corticosteroids may improve the compromised anti-inflammatory properties of corticosteroids in COPD. We analyzed differential and combination anti-inflammatory effects of dexamethasone and roflumilast N-oxide in human bronchial epithelial cells (HBECs) stimulated with viral toll like receptor (TLR) agonists.
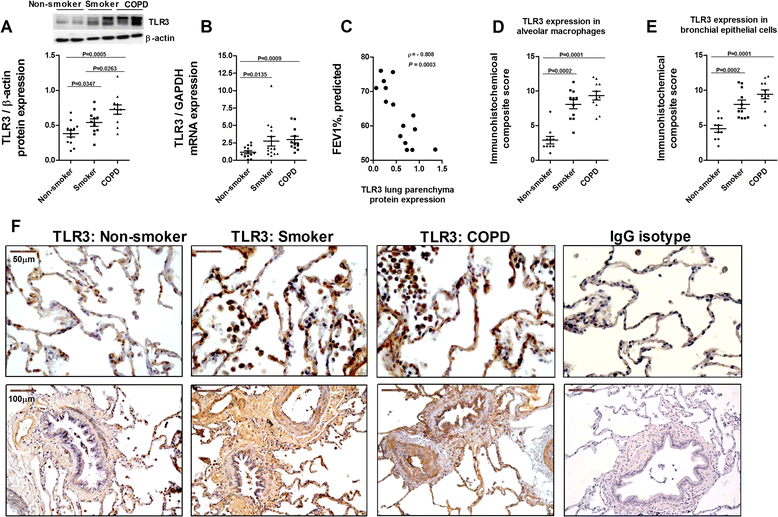
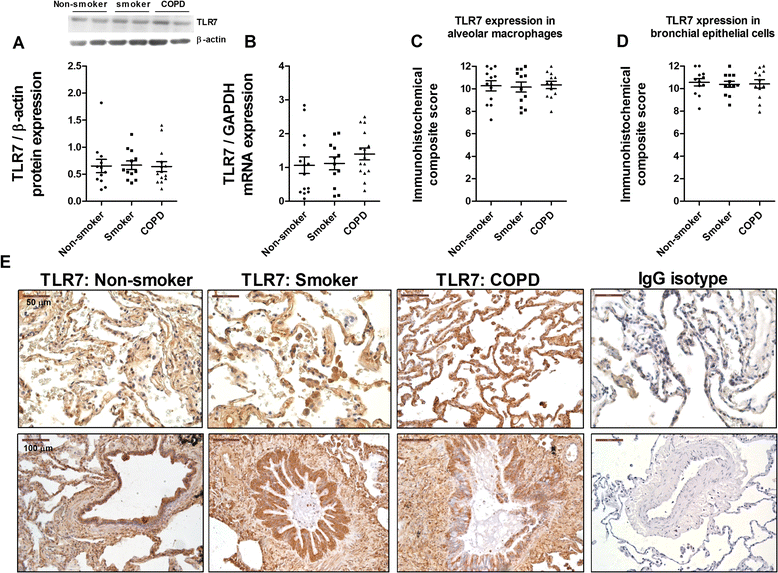
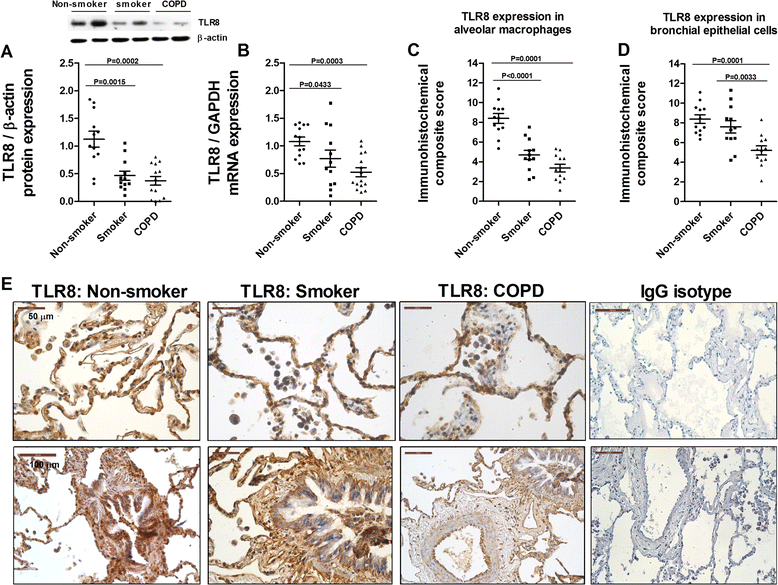
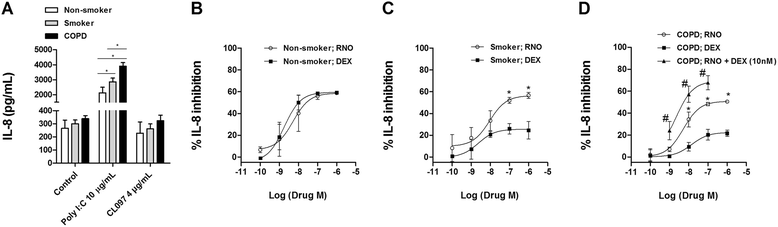
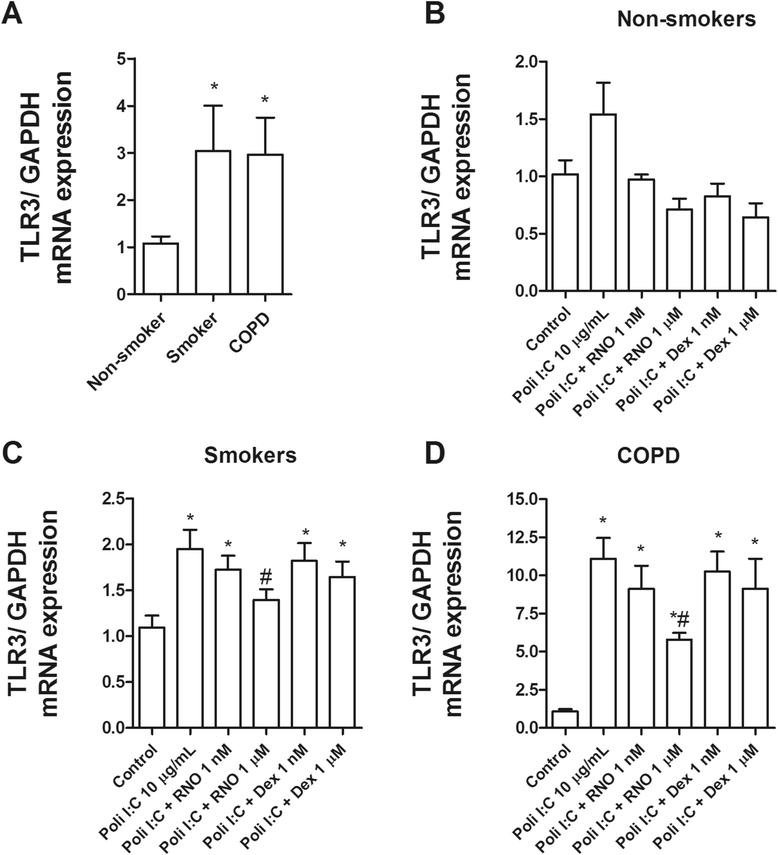
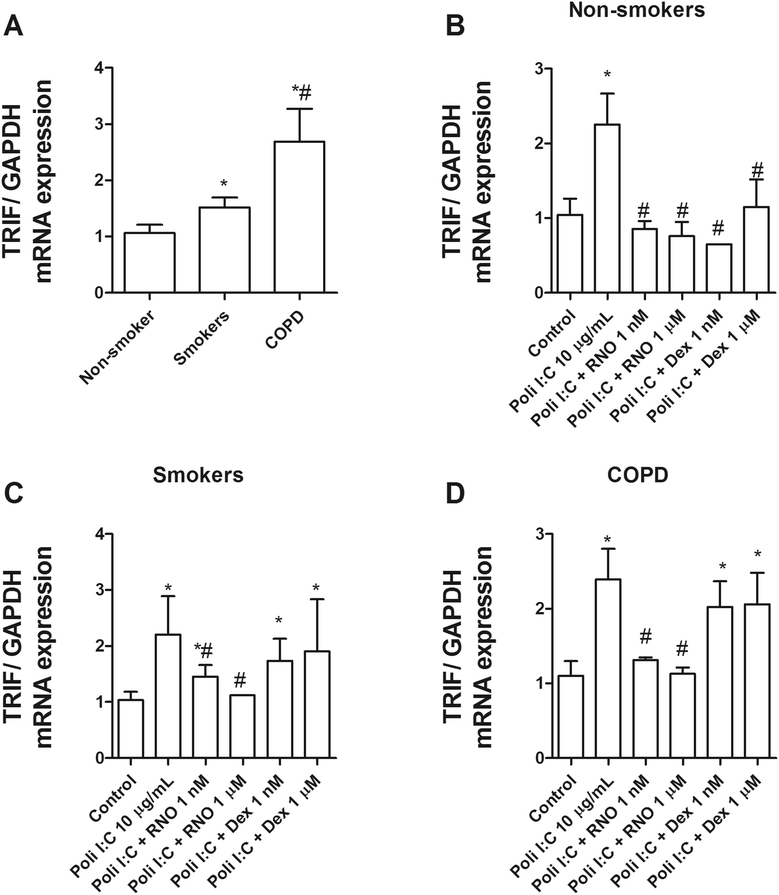
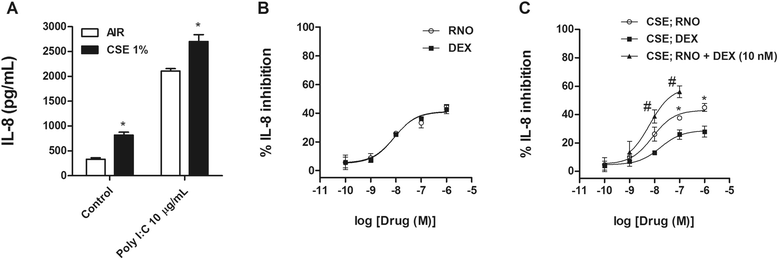
Methods: Lung tissue and HBECs were isolated from healthy (n = 15), smokers (n = 12) and smokers with COPD (15). TLR3 expression was measured in lung tissue and in HBECs. IL-8 secretion was measured in cell cultures after TLR3 stimulation with poly I:C 10 μg/mL.
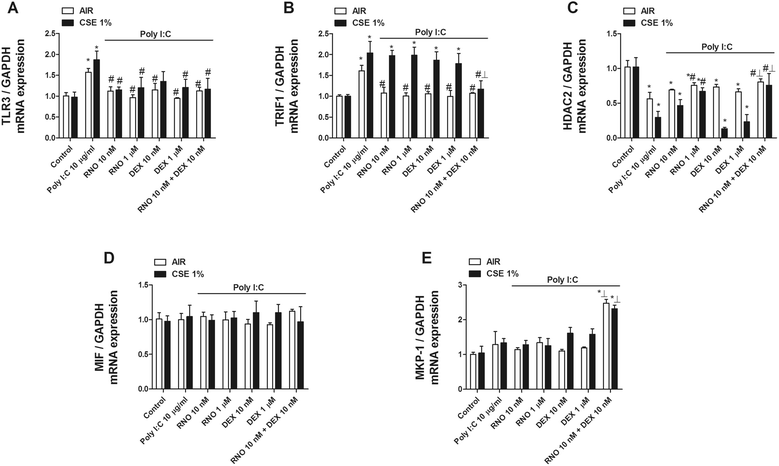
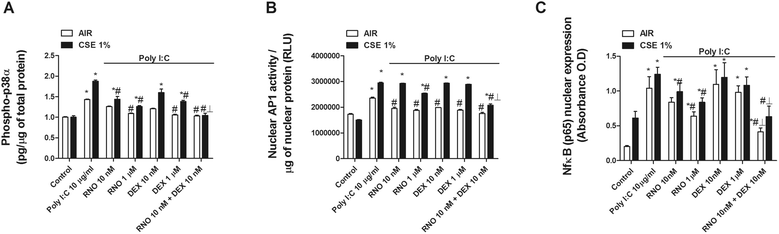
Results: We found that TLR3 expression was increased by 1.95 fold (protein) and 2.5 fold (mRNA) in lung tissues from smokers with COPD and inversely correlated with lung function. The TLR3 agonist poly I:C 10 μg/mL increased the IL-8 release in HBECs that was poorly inhibited by dexamethasone in smokers (24.5%) and smokers with COPD (21.6%). In contrast, roflumilast showed similar inhibitory effects on IL-8 release in healthy (58.8%), smokers (56.6%) and smokers with COPD (50.5%). The combination of roflumilast N-oxide and dexamethasone showed additive inhibitory effects. Mechanistically, roflumilast N-oxide when combined with dexamethasone increased the expression of MKP1, and enhanced the inhibitory effects on phospho-p38, AP1 and NFκB activities which may explain the additive anti-inflammatory effects.
Conclusions: Altogether, our data provide in vitro evidence for a possible clinical utility to add roflumilast on top of inhaled corticosteroid in COPD.
Figures
Similar articles
-
Roflumilast N-oxide reverses corticosteroid resistance in neutrophils from patients with chronic obstructive pulmonary disease.J Allergy Clin Immunol. 2014 Aug;134(2):314-22. doi: 10.1016/j.jaci.2014.02.001. Epub 2014 Mar 15. J Allergy Clin Immunol. 2014. PMID: 24636089
-
Roflumilast N-oxide inhibits bronchial epithelial to mesenchymal transition induced by cigarette smoke in smokers with COPD.Pulm Pharmacol Ther. 2014 Aug;28(2):138-48. doi: 10.1016/j.pupt.2014.02.001. Epub 2014 Feb 11. Pulm Pharmacol Ther. 2014. PMID: 24525294
-
Glucocorticoid Receptor α Mediates Roflumilast's Ability to Restore Dexamethasone Sensitivity in COPD.Int J Chron Obstruct Pulmon Dis. 2020 Jan 14;15:125-134. doi: 10.2147/COPD.S230188. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32021151 Free PMC article.
-
The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease.Pulm Pharmacol Ther. 2010 Aug;23(4):235-56. doi: 10.1016/j.pupt.2010.03.011. Epub 2010 Apr 7. Pulm Pharmacol Ther. 2010. PMID: 20381629 Review.
-
Pharmacokinetic evaluation of roflumilast.Expert Opin Drug Metab Toxicol. 2011 Dec;7(12):1577-91. doi: 10.1517/17425255.2011.632409. Epub 2011 Nov 8. Expert Opin Drug Metab Toxicol. 2011. PMID: 22059647 Review.
Cited by
-
Changes in Pulmonary Microenvironment Aids Lung Metastasis of Breast Cancer.Front Oncol. 2022 May 26;12:860932. doi: 10.3389/fonc.2022.860932. eCollection 2022. Front Oncol. 2022. PMID: 35719975 Free PMC article. Review.
-
Extracellular ATP is involved in dsRNA-induced MUC5AC production via P2Y2R in human airway epithelium.Respir Res. 2016 Sep 27;17(1):121. doi: 10.1186/s12931-016-0438-0. Respir Res. 2016. PMID: 27677339 Free PMC article.
-
New putative insights into neprilysin (NEP)-dependent pharmacotherapeutic role of roflumilast in treating COVID-19.Eur J Pharmacol. 2020 Dec 15;889:173615. doi: 10.1016/j.ejphar.2020.173615. Epub 2020 Oct 1. Eur J Pharmacol. 2020. PMID: 33011243 Free PMC article. Review.
-
Differential response to roflumilast in patients with chronic obstructive pulmonary disease: real-world evidence.J Thorac Dis. 2024 Feb 29;16(2):1338-1349. doi: 10.21037/jtd-23-1129. Epub 2024 Feb 27. J Thorac Dis. 2024. PMID: 38505074 Free PMC article.
-
Differential anti-inflammatory effects of budesonide and a p38 MAPK inhibitor AZD7624 on COPD pulmonary cells.Int J Chron Obstruct Pulmon Dis. 2018 Apr 19;13:1279-1288. doi: 10.2147/COPD.S159936. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29719383 Free PMC article.
References
-
- Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast–a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23(4):235–56. doi: 10.1016/j.pupt.2010.03.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

