Intracrine Androgens and AKR1C3 Activation Confer Resistance to Enzalutamide in Prostate Cancer
- PMID: 25649766
- PMCID: PMC4383695
- DOI: 10.1158/0008-5472.CAN-14-3080
Intracrine Androgens and AKR1C3 Activation Confer Resistance to Enzalutamide in Prostate Cancer
Abstract
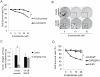
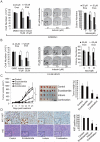
The introduction of enzalutamide and abiraterone has led to improvement in the treatment of metastatic castration-resistant prostate cancer. However, acquired resistance to enzalutamide and abiraterone therapies frequently develops within a short period in many patients. In the present study, we developed enzalutamide-resistant prostate cancer cells in an effort to understand the mechanisms of resistance. Global gene-expression analysis showed that the steroid biosynthesis pathway is activated in enzalutamide-resistant prostate cancer cells. One of the crucial steroidogenic enzymes, AKR1C3, was significantly elevated in enzalutamide-resistant cells. In addition, AKR1C3 is highly expressed in metastatic and recurrent prostate cancer and in enzalutamide-resistant prostate xenograft tumors. LC/MS analysis of the steroid metabolites revealed that androgen precursors such as cholesterol, DHEA and progesterone, as well as androgens are highly upregulated in enzalutamide-resistant prostate cancer cells compared to the parental cells. Knockdown of AKR1C3 expression by shRNA or inhibition of AKR1C3 enzymatic activity by indomethacin resensitized enzalutamide-resistant prostate cancer cells to enzalutamide treatment both in vitro and in vivo. In contrast, overexpression of AKR1C3 confers resistance to enzalutamide. Furthermore, the combination of indomethacin and enzalutamide resulted in significant inhibition of enzalutamide-resistant tumor growth. These results suggest that AKR1C3 activation is a critical resistance mechanism associated with enzalutamide resistance; targeting intracrine androgens and AKR1C3 will overcome enzalutamide resistance and improve survival of advanced prostate cancer patients.
©2015 American Association for Cancer Research.
Figures



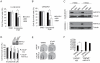
Comment in
-
Prostate cancer: Breaking AKR1C3-mediated enzalutamide resistance by inhibiting androgen synthesis.Nat Rev Urol. 2015 Mar;12(3):124. doi: 10.1038/nrurol.2015.31. Epub 2015 Feb 24. Nat Rev Urol. 2015. PMID: 25708577 No abstract available.
Similar articles
-
Inhibition of AKR1C3 Activation Overcomes Resistance to Abiraterone in Advanced Prostate Cancer.Mol Cancer Ther. 2017 Jan;16(1):35-44. doi: 10.1158/1535-7163.MCT-16-0186. Epub 2016 Oct 28. Mol Cancer Ther. 2017. PMID: 27794047 Free PMC article.
-
AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects and Potentiates Enzalutamide in Combination Therapy in Prostate Adenocarcinoma Cells.Mol Cancer Ther. 2018 Sep;17(9):1833-1845. doi: 10.1158/1535-7163.MCT-17-1023. Epub 2018 Jun 11. Mol Cancer Ther. 2018. PMID: 29891491
-
Overexpression of aldo-keto reductase 1C3 (AKR1C3) in LNCaP cells diverts androgen metabolism towards testosterone resulting in resistance to the 5α-reductase inhibitor finasteride.J Steroid Biochem Mol Biol. 2012 May;130(1-2):7-15. doi: 10.1016/j.jsbmb.2011.12.012. Epub 2012 Jan 12. J Steroid Biochem Mol Biol. 2012. PMID: 22265960 Free PMC article.
-
The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer.Chem Biol Interact. 2015 Jun 5;234:332-8. doi: 10.1016/j.cbi.2014.12.012. Epub 2014 Dec 13. Chem Biol Interact. 2015. PMID: 25514466 Free PMC article. Review.
-
Inhibitors of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3): overview and structural insights.J Steroid Biochem Mol Biol. 2011 May;125(1-2):95-104. doi: 10.1016/j.jsbmb.2010.11.004. Epub 2010 Nov 16. J Steroid Biochem Mol Biol. 2011. PMID: 21087665 Free PMC article. Review.
Cited by
-
Proteostasis perturbation of N-Myc leveraging HSP70 mediated protein turnover improves treatment of neuroendocrine prostate cancer.Nat Commun. 2024 Aug 5;15(1):6626. doi: 10.1038/s41467-024-50459-x. Nat Commun. 2024. PMID: 39103353 Free PMC article.
-
Stromal-derived MAOB promotes prostate cancer growth and progression.Sci Adv. 2024 Feb 9;10(6):eadi4935. doi: 10.1126/sciadv.adi4935. Epub 2024 Feb 9. Sci Adv. 2024. PMID: 38335292 Free PMC article.
-
Targeting backdoor androgen synthesis through AKR1C3 inhibition: A presurgical hormonal ablative neoadjuvant trial in high-risk localized prostate cancer.Prostate. 2021 May;81(7):418-426. doi: 10.1002/pros.24118. Epub 2021 Mar 23. Prostate. 2021. PMID: 33755225 Free PMC article.
-
Inhibition of AKR1C3 Activation Overcomes Resistance to Abiraterone in Advanced Prostate Cancer.Mol Cancer Ther. 2017 Jan;16(1):35-44. doi: 10.1158/1535-7163.MCT-16-0186. Epub 2016 Oct 28. Mol Cancer Ther. 2017. PMID: 27794047 Free PMC article.
-
Synthetic lethal combination of CHK1 and WEE1 inhibition for treatment of castration-resistant prostate cancer.Res Sq [Preprint]. 2023 Nov 8:rs.3.rs-3564450. doi: 10.21203/rs.3.rs-3564450/v1. Res Sq. 2023. Update in: Oncogene. 2024 Mar;43(11):789-803. doi: 10.1038/s41388-024-02939-z. PMID: 37987002 Free PMC article. Updated. Preprint.
References
-
- Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer discovery. 2013;3:1030–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

