Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785
- PMID: 25648083
- PMCID: PMC4326364
- DOI: 10.1186/s12866-015-0347-2
Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785
Abstract
Background: The bacterial cell surface is a crucial factor in cell-cell and cell-host interactions. Lactobacillus johnsonii FI9785 produces an exopolysaccharide (EPS) layer whose quantity and composition is altered in mutants that harbour genetic changes in their eps gene clusters. We have assessed the effect of changes in EPS production on cell surface characteristics that may affect the ability of L. johnsonii to colonise the poultry host and exclude pathogens.
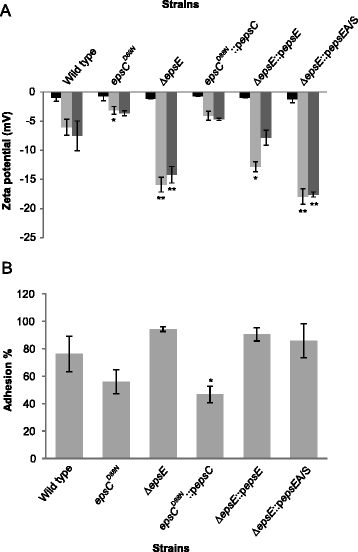
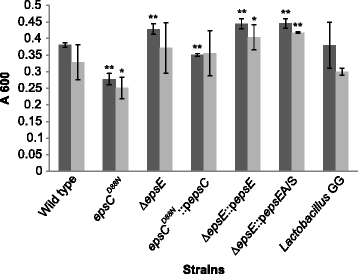
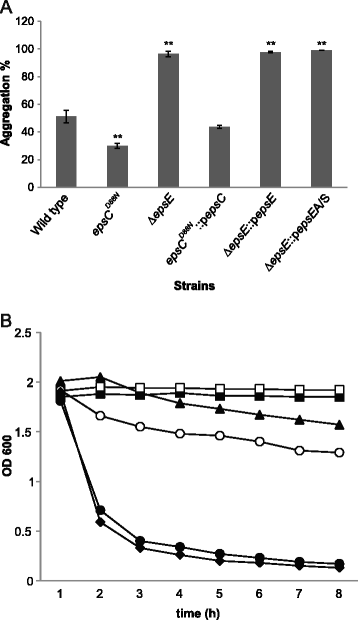
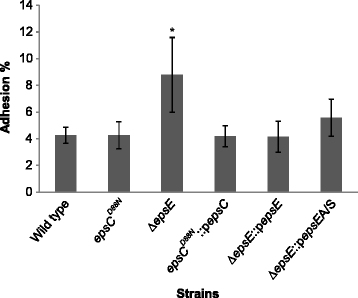
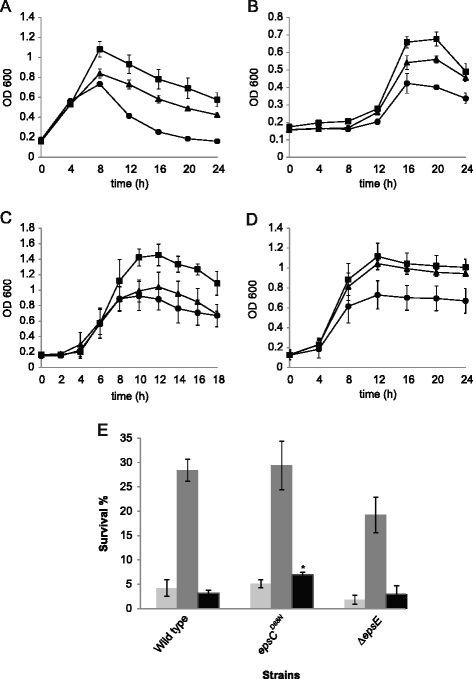
Results: Analysis of physicochemical cell surface characteristics reflected by Zeta potential and adhesion to hexadecane showed that an increase in EPS gave a less negative, more hydrophilic surface and reduced autoaggregation. Autoaggregation was significantly higher in mutants that have reduced EPS, indicating that EPS can mask surface structures responsible for cell-cell interactions. EPS also affected biofilm formation, but here the quantity of EPS produced was not the only determinant. A reduction in EPS production increased bacterial adhesion to chicken gut explants, but made the bacteria less able to survive some stresses.
Conclusions: This study showed that manipulation of EPS production in L. johnsonii FI9785 can affect properties which may improve its performance as a competitive exclusion agent, but that positive changes in adhesion may be compromised by a reduction in the ability to survive stress.
Figures
Similar articles
-
Impact of exopolysaccharide production on functional properties of some Lactobacillus salivarius strains.Arch Microbiol. 2015 Nov;197(9):1041-9. doi: 10.1007/s00203-015-1141-0. Epub 2015 Aug 13. Arch Microbiol. 2015. PMID: 26267164
-
Structure and biosynthesis of two exopolysaccharides produced by Lactobacillus johnsonii FI9785.J Biol Chem. 2013 Nov 1;288(44):31938-51. doi: 10.1074/jbc.M113.507418. Epub 2013 Sep 9. J Biol Chem. 2013. PMID: 24019531 Free PMC article.
-
Spontaneous mutation reveals influence of exopolysaccharide on Lactobacillus johnsonii surface characteristics.PLoS One. 2013;8(3):e59957. doi: 10.1371/journal.pone.0059957. Epub 2013 Mar 27. PLoS One. 2013. PMID: 23544114 Free PMC article.
-
Bacterial exopolysaccharides as emerging bioactive macromolecules: from fundamentals to applications.Res Microbiol. 2023 May;174(4):104024. doi: 10.1016/j.resmic.2022.104024. Epub 2022 Dec 30. Res Microbiol. 2023. PMID: 36587857 Review.
-
Interactions of Surface Exopolysaccharides From Bifidobacterium and Lactobacillus Within the Intestinal Environment.Front Microbiol. 2018 Oct 11;9:2426. doi: 10.3389/fmicb.2018.02426. eCollection 2018. Front Microbiol. 2018. PMID: 30364185 Free PMC article. Review.
Cited by
-
The Immunomodulatory Properties of β-2,6 Fructans: A Comprehensive Review.Nutrients. 2021 Apr 15;13(4):1309. doi: 10.3390/nu13041309. Nutrients. 2021. PMID: 33921025 Free PMC article. Review.
-
Development of an in vitro Assay, Based on the BioFilm Ring Test®, for Rapid Profiling of Biofilm-Growing Bacteria.Front Microbiol. 2016 Sep 21;7:1429. doi: 10.3389/fmicb.2016.01429. eCollection 2016. Front Microbiol. 2016. PMID: 27708625 Free PMC article.
-
A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition.Int J Mol Sci. 2022 Jan 3;23(1):513. doi: 10.3390/ijms23010513. Int J Mol Sci. 2022. PMID: 35008939 Free PMC article.
-
Biodegradation of polycyclic aromatic hydrocarbons by an acidophilic Stenotrophomonas maltophilia strain AJH1 isolated from a mineral mining site in Saudi Arabia.Extremophiles. 2017 Jan;21(1):163-174. doi: 10.1007/s00792-016-0892-0. Epub 2016 Nov 16. Extremophiles. 2017. PMID: 27848015
-
Genotypic and phenotypic diversity of Lactobacillus rhamnosus clinical isolates, their comparison with strain GG and their recognition by complement system.PLoS One. 2017 May 11;12(5):e0176739. doi: 10.1371/journal.pone.0176739. eCollection 2017. PLoS One. 2017. PMID: 28493885 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

