Understanding opioid reward
- PMID: 25637939
- PMCID: PMC4385443
- DOI: 10.1016/j.tins.2015.01.002
Understanding opioid reward
Abstract
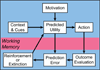
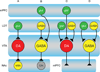
Opioids are the most potent analgesics in clinical use; however, their powerful rewarding properties can lead to addiction. The scientific challenge is to retain analgesic potency while limiting the development of tolerance, dependence, and addiction. Both rewarding and analgesic actions of opioids depend upon actions at the mu opioid (MOP) receptor. Systemic opioid reward requires MOP receptor function in the midbrain ventral tegmental area (VTA) which contains dopaminergic neurons. VTA dopaminergic neurons are implicated in various aspects of reward including reward prediction error, working memory, and incentive salience. It is now clear that subsets of VTA neurons have different pharmacological properties and participate in separate circuits. The degree to which MOP receptor agonists act on different VTA circuits depends upon the behavioral state of the animal, which can be altered by manipulations such as food deprivation or prior exposure to MOP receptor agonists.
Keywords: VTA; addiction; midbrain; morphine; mu opioid receptor.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Direct bidirectional μ-opioid control of midbrain dopamine neurons.J Neurosci. 2014 Oct 29;34(44):14707-16. doi: 10.1523/JNEUROSCI.2144-14.2014. J Neurosci. 2014. PMID: 25355223 Free PMC article.
-
Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons.J Neurosci. 2013 Apr 10;33(15):6454-9. doi: 10.1523/JNEUROSCI.0178-13.2013. J Neurosci. 2013. PMID: 23575843 Free PMC article.
-
Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits.Pharmacol Biochem Behav. 2021 Jan;200:173072. doi: 10.1016/j.pbb.2020.173072. Epub 2020 Nov 20. Pharmacol Biochem Behav. 2021. PMID: 33227308 Free PMC article. Review.
-
Increased gabaergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice.Neuroscience. 2005;130(2):359-67. doi: 10.1016/j.neuroscience.2004.10.002. Neuroscience. 2005. PMID: 15664692
-
The Dopaminergic System in the Ventral Tegmental Area Contributes to Morphine Analgesia and Tolerance.Neuroscience. 2023 Sep 1;527:74-83. doi: 10.1016/j.neuroscience.2023.05.026. Epub 2023 Jun 5. Neuroscience. 2023. PMID: 37286162 Review.
Cited by
-
Mu opioid receptors on vGluT2-expressing glutamatergic neurons modulate opioid reward.Addict Biol. 2021 May;26(3):e12942. doi: 10.1111/adb.12942. Epub 2020 Jul 20. Addict Biol. 2021. PMID: 32686251 Free PMC article.
-
Signaling mechanisms of μ-opioid receptor (MOR) in the hippocampus: disinhibition versus astrocytic glutamate regulation.Cell Mol Life Sci. 2021 Jan;78(2):415-426. doi: 10.1007/s00018-020-03595-8. Epub 2020 Jul 15. Cell Mol Life Sci. 2021. PMID: 32671427 Free PMC article. Review.
-
Reward Enhances Pain Discrimination in Humans.Psychol Sci. 2020 Sep;31(9):1191-1199. doi: 10.1177/0956797620939588. Epub 2020 Aug 20. Psychol Sci. 2020. PMID: 32818387 Free PMC article.
-
Episodic Ethanol Exposure in Adolescent Rats Causes Residual Alterations in Endogenous Opioid Peptides.Front Psychiatry. 2018 Sep 10;9:425. doi: 10.3389/fpsyt.2018.00425. eCollection 2018. Front Psychiatry. 2018. PMID: 30250435 Free PMC article.
-
Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects.Neuropsychopharmacology. 2019 Jul;44(8):1415-1424. doi: 10.1038/s41386-018-0284-5. Epub 2018 Nov 27. Neuropsychopharmacology. 2019. PMID: 30555159 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

