GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6
- PMID: 25637055
- PMCID: PMC4349277
- DOI: 10.3324/haematol.2014.117945
GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6
Abstract
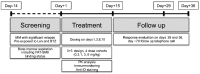
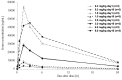
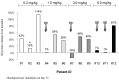
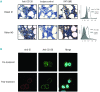
The primary objective of this phase 1 study was to evaluate the safety and tolerability of the anti-glucose regulated protein 78 monoclonal immunoglobulin M antibody PAT-SM6 in subjects with relapsed or refractory multiple myeloma. Twelve heavily pretreated patients received four intravenous infusions of PAT-SM6 at doses of 0.3, 1, 3, and 6 mg/kg within 2 weeks. Efficacy, pharmacokinetics and immunogenicity were followed up until the end of the trial (day 36). In addition, immune cell patterns in peripheral blood were assessed by flow cytometry and glucose regulated protein 78 expression status was evaluated in bone marrow specimens by immunohistochemistry and flow cytometry at screening. All doses administered were found to be safe and well tolerated; the maximum tolerated dose was not reached. The most common treatment emergent adverse event was leukopenia (grades 1 and 2) in eight out of the 12 multiple myeloma patients. Pharmacokinetic analysis demonstrated dose-proportional increases in drug serum concentration. The terminal half-life ranged from 5.86 to 8.41 h, the apparent volume of distribution ranged from 101 to 150 mL/kg, and clearance ranged from 8.11 to 16.1 mL/h/kg. All patients showed glucose regulated protein 78 surface expression on multiple myeloma cells. Four out of the 12 patients (33.3 %) had stable disease, according to the International Myeloma Working Group criteria, after PAT-SM6 treatment across the doses 1, 3 and 6 mg/kg. In summary, single-agent PAT-SM6 was well tolerated with modest clinical activity in relapsed or refractory multiple myeloma. Further trials exploring the combination of PAT-SM6 with existing myeloma therapies are planned.
Trial registration: clinicaltrials.gov identifier: NCT01727778.
Copyright© Ferrata Storti Foundation.
Figures
Similar articles
-
A GRP78-Directed Monoclonal Antibody Recaptures Response in Refractory Multiple Myeloma with Extramedullary Involvement.Clin Cancer Res. 2016 Sep 1;22(17):4341-9. doi: 10.1158/1078-0432.CCR-15-3111. Epub 2016 Mar 30. Clin Cancer Res. 2016. PMID: 27029491
-
The natural human IgM antibody PAT-SM6 induces apoptosis in primary human multiple myeloma cells by targeting heat shock protein GRP78.PLoS One. 2013 May 7;8(5):e63414. doi: 10.1371/journal.pone.0063414. Print 2013. PLoS One. 2013. PMID: 23667612 Free PMC article.
-
Early development of PAT-SM6 for the treatment of melanoma.Melanoma Res. 2013 Aug;23(4):264-75. doi: 10.1097/CMR.0b013e328362cbc8. Melanoma Res. 2013. PMID: 23728394
-
Treatment of multiple myeloma by antibody mediated immunotherapy and induction of myeloma selective antigens.Ann Oncol. 2000;11 Suppl 1:107-11. Ann Oncol. 2000. PMID: 10707790 Review.
-
Practical Considerations for the Use of Daratumumab, a Novel CD38 Monoclonal Antibody, in Myeloma.Drugs. 2016 May;76(8):853-67. doi: 10.1007/s40265-016-0573-4. Drugs. 2016. PMID: 27113582 Review.
Cited by
-
Structure, Function, and Therapeutic Use of IgM Antibodies.Antibodies (Basel). 2020 Oct 13;9(4):53. doi: 10.3390/antib9040053. Antibodies (Basel). 2020. PMID: 33066119 Free PMC article. Review.
-
Similarities and Differences of Hsp70, hsc70, Grp78 and Mortalin as Cancer Biomarkers and Drug Targets.Cells. 2021 Nov 3;10(11):2996. doi: 10.3390/cells10112996. Cells. 2021. PMID: 34831218 Free PMC article. Review.
-
Monoclonal Antibody Therapies for Hematological Malignancies: Not Just Lineage-Specific Targets.Front Immunol. 2018 Jan 17;8:1936. doi: 10.3389/fimmu.2017.01936. eCollection 2017. Front Immunol. 2018. PMID: 29387053 Free PMC article. Review.
-
Immunologic approaches for the treatment of multiple myeloma.Cancer Treat Rev. 2017 Apr;55:190-199. doi: 10.1016/j.ctrv.2017.03.010. Epub 2017 Apr 6. Cancer Treat Rev. 2017. PMID: 28431262 Free PMC article. Review.
-
CAR-Based Immunotherapy of Solid Tumours-A Survey of the Emerging Targets.Cancers (Basel). 2023 Feb 11;15(4):1171. doi: 10.3390/cancers15041171. Cancers (Basel). 2023. PMID: 36831514 Free PMC article. Review.
References
-
- Danylesko I, Beider K, Shimoni A, Nagler A. Monoclonal antibody-based immunotherapy for multiple myeloma. Immunotherapy. 2012;4(9):919–938. - PubMed
-
- de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

