The chemokine system in innate immunity
- PMID: 25635046
- PMCID: PMC4448619
- DOI: 10.1101/cshperspect.a016303
The chemokine system in innate immunity
Abstract
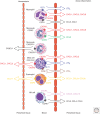
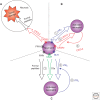
Chemokines are chemotactic cytokines that control the migration and positioning of immune cells in tissues and are critical for the function of the innate immune system. Chemokines control the release of innate immune cells from the bone marrow during homeostasis as well as in response to infection and inflammation. They also recruit innate immune effectors out of the circulation and into the tissue where, in collaboration with other chemoattractants, they guide these cells to the very sites of tissue injury. Chemokine function is also critical for the positioning of innate immune sentinels in peripheral tissue and then, following innate immune activation, guiding these activated cells to the draining lymph node to initiate and imprint an adaptive immune response. In this review, we will highlight recent advances in understanding how chemokine function regulates the movement and positioning of innate immune cells at homeostasis and in response to acute inflammation, and then we will review how chemokine-mediated innate immune cell trafficking plays an essential role in linking the innate and adaptive immune responses.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Chemokines and chemokine receptors: positioning cells for host defense and immunity.Annu Rev Immunol. 2014;32:659-702. doi: 10.1146/annurev-immunol-032713-120145. Annu Rev Immunol. 2014. PMID: 24655300 Review.
-
Chemokine regulation of innate lymphoid cell tissue distribution and function.Cytokine Growth Factor Rev. 2018 Aug;42:47-55. doi: 10.1016/j.cytogfr.2018.02.003. Epub 2018 Feb 14. Cytokine Growth Factor Rev. 2018. PMID: 29472011 Review.
-
Chemokines: key players in innate and adaptive immunity.J Invest Dermatol. 2005 Oct;125(4):615-28. doi: 10.1111/j.0022-202X.2005.23841.x. J Invest Dermatol. 2005. PMID: 16185259 Review.
-
Chemokine-guided cell positioning in the lymph node orchestrates the generation of adaptive immune responses.Curr Opin Cell Biol. 2015 Oct;36:1-6. doi: 10.1016/j.ceb.2015.05.003. Epub 2015 Jun 8. Curr Opin Cell Biol. 2015. PMID: 26067148 Free PMC article. Review.
-
The role of chemokines in linking innate and adaptive immunity.Curr Opin Immunol. 2002 Feb;14(1):129-35. doi: 10.1016/s0952-7915(01)00308-9. Curr Opin Immunol. 2002. PMID: 11790543 Review.
Cited by
-
Immune phenotypes that are associated with subsequent COVID-19 severity inferred from post-recovery samples.Nat Commun. 2022 Nov 25;13(1):7255. doi: 10.1038/s41467-022-34638-2. Nat Commun. 2022. PMID: 36433939 Free PMC article.
-
Microglia Ontology and Signaling.Front Cell Dev Biol. 2016 Jun 29;4:72. doi: 10.3389/fcell.2016.00072. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27446922 Free PMC article. Review.
-
Prognostic value of CXCL17 and CXCR8 expression in patients with colon cancer.Oncol Lett. 2020 Sep;20(3):2711-2720. doi: 10.3892/ol.2020.11819. Epub 2020 Jul 7. Oncol Lett. 2020. PMID: 32782587 Free PMC article.
-
A tyrosine kinase inhibitor-induced interferon response positively associates with clinical response in EGFR-mutant lung cancer.NPJ Precis Oncol. 2021 May 17;5(1):41. doi: 10.1038/s41698-021-00181-4. NPJ Precis Oncol. 2021. PMID: 34001994 Free PMC article.
-
Acute stress induces an inflammation dominated by innate immunity represented by neutrophils in mice.Front Immunol. 2022 Sep 29;13:1014296. doi: 10.3389/fimmu.2022.1014296. eCollection 2022. Front Immunol. 2022. PMID: 36248830 Free PMC article.
References
-
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. 2003. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19: 257–267. - PubMed
-
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
