Reduced granulation tissue and wound strength in the absence of α11β1 integrin
- PMID: 25634355
- PMCID: PMC4407012
- DOI: 10.1038/jid.2015.24
Reduced granulation tissue and wound strength in the absence of α11β1 integrin
Abstract
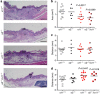
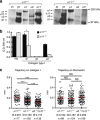
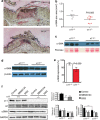
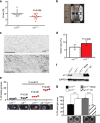
Previous wound healing studies have failed to define a role for either α1β1 or α2β1 integrin in fibroblast-mediated wound contraction, suggesting the involvement of another collagen receptor in this process. Our previous work demonstrated that the integrin subunit α11 is highly induced during wound healing both at the mRNA and protein level, prompting us to investigate and dissect the role of the integrin α11β1 during this process. Therefore, we used mice with a global ablation of either α2 or α11 or both integrin subunits and investigated the repair of excisional wounds. Analyses of wounds demonstrated that α11β1 deficiency results in reduced granulation tissue formation and impaired wound contraction, independently of the presence of α2β1. Our combined in vivo and in vitro data further demonstrate that dermal fibroblasts lacking α11β1 are unable to efficiently convert to myofibroblasts, resulting in scar tissue with compromised tensile strength. Moreover, we suggest that the reduced stability of the scar is a consequence of poor collagen remodeling in α11(-/-) wounds associated with defective transforming growth factor-β-dependent JNK signaling.
Figures

Similar articles
-
Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor.Mol Cell Biol. 2007 Jun;27(12):4306-16. doi: 10.1128/MCB.00041-07. Epub 2007 Apr 9. Mol Cell Biol. 2007. PMID: 17420280 Free PMC article.
-
The mesenchymal alpha11beta1 integrin attenuates PDGF-BB-stimulated chemotaxis of embryonic fibroblasts on collagens.Dev Biol. 2004 Jun 15;270(2):427-42. doi: 10.1016/j.ydbio.2004.03.006. Dev Biol. 2004. PMID: 15183724
-
Integrin α11β1: a major collagen receptor on fibroblastic cells.Adv Exp Med Biol. 2014;819:73-83. doi: 10.1007/978-94-017-9153-3_5. Adv Exp Med Biol. 2014. PMID: 25023168 Review.
-
Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization.J Invest Dermatol. 2007 Feb;127(2):467-78. doi: 10.1038/sj.jid.5700546. Epub 2006 Sep 14. J Invest Dermatol. 2007. PMID: 16977325
-
The integrin-collagen connection--a glue for tissue repair?J Cell Sci. 2016 Feb 15;129(4):653-64. doi: 10.1242/jcs.180992. Epub 2016 Feb 8. J Cell Sci. 2016. PMID: 26857815 Review.
Cited by
-
Collagen Assembly at the Cell Surface: Dogmas Revisited.Cells. 2021 Mar 16;10(3):662. doi: 10.3390/cells10030662. Cells. 2021. PMID: 33809734 Free PMC article. Review.
-
LOXL1 Is Regulated by Integrin α11 and Promotes Non-Small Cell Lung Cancer Tumorigenicity.Cancers (Basel). 2019 May 22;11(5):705. doi: 10.3390/cancers11050705. Cancers (Basel). 2019. PMID: 31121900 Free PMC article.
-
TGFB1 is secreted through an unconventional pathway dependent on the autophagic machinery and cytoskeletal regulators.Autophagy. 2018;14(3):465-486. doi: 10.1080/15548627.2017.1422850. Epub 2018 Mar 11. Autophagy. 2018. PMID: 29297744 Free PMC article.
-
Fibroblast and myofibroblast activation in normal tissue repair and fibrosis.Nat Rev Mol Cell Biol. 2024 Aug;25(8):617-638. doi: 10.1038/s41580-024-00716-0. Epub 2024 Apr 8. Nat Rev Mol Cell Biol. 2024. PMID: 38589640 Review.
-
Increased dermal collagen bundle alignment in systemic sclerosis is associated with a cell migration signature and role of Arhgdib in directed fibroblast migration on aligned ECMs.PLoS One. 2017 Jun 29;12(6):e0180751. doi: 10.1371/journal.pone.0180751. eCollection 2017. PLoS One. 2017. PMID: 28662216 Free PMC article.
References
-
- Barczyk MM, Lu N, Popova SN, et al. alpha11beta1 integrin-mediated MMP-13-dependent collagen lattice contraction by fibroblasts: Evidence for integrin-coordinated collagen proteolysis. J Cell Physiol. 2013;228:1108–1119. - PubMed
-
- Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16:472–479. - PubMed
-
- Egbert M, Ruetze M, Sattler M, et al. The matricellular protein periostin contributes to proper collagen function and is downregulated during skin aging. J Dermatol Sci. 2014;73:40–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

