A new window into the molecular physiology of membrane proteins
- PMID: 25630257
- PMCID: PMC4303381
- DOI: 10.1113/jphysiol.2014.283150
A new window into the molecular physiology of membrane proteins
Abstract
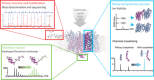
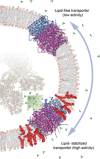
Integral membrane proteins comprise ∼25% of the human proteome. Yet, our understanding of their molecular physiology is still in its infancy. This can be attributed to two factors: the experimental challenges that arise from the difficult chemical nature of membrane proteins, and the unclear relationship between their activity and their native environment. New approaches are therefore required to address these challenges. Recent developments in mass spectrometry have shown that it is possible to study membrane proteins in a solvent-free environment and provide detailed insights into complex interactions, ligand binding and folding processes. Interestingly, not only detergent micelles but also lipid bilayer nanodiscs or bicelles can serve as a means for the gentle desolvation of membrane proteins in the gas phase. In this manner, as well as by direct addition of lipids, it is possible to study the effects of different membrane components on the structure and function of the protein components allowing us to add functional data to the least accessible part of the proteome.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures
Comment in
-
Lipids Can Make Them Stick Together.Trends Biochem Sci. 2017 May;42(5):329-330. doi: 10.1016/j.tibs.2017.03.001. Epub 2017 Mar 28. Trends Biochem Sci. 2017. PMID: 28363673
Similar articles
-
Nanodiscs and SILAC-based mass spectrometry to identify a membrane protein interactome.J Proteome Res. 2012 Feb 3;11(2):1454-9. doi: 10.1021/pr200846y. Epub 2011 Dec 22. J Proteome Res. 2012. PMID: 22129326
-
Native Mass Spectrometry for the Characterization of Structure and Interactions of Membrane Proteins.Methods Mol Biol. 2017;1635:205-232. doi: 10.1007/978-1-4939-7151-0_11. Methods Mol Biol. 2017. PMID: 28755371
-
Probing the Lipid Annular Belt by Gas-Phase Dissociation of Membrane Proteins in Nanodiscs.Angew Chem Int Ed Engl. 2016 Jan 11;55(2):550-4. doi: 10.1002/anie.201508289. Epub 2015 Nov 23. Angew Chem Int Ed Engl. 2016. PMID: 26594028 Free PMC article.
-
Different modes of lipid binding to membrane proteins probed by mass spectrometry.J Am Chem Soc. 2015 Apr 29;137(16):5240-7. doi: 10.1021/jacs.5b00420. Epub 2015 Apr 16. J Am Chem Soc. 2015. PMID: 25860341 Review.
-
Extending native mass spectrometry approaches to integral membrane proteins.Biol Chem. 2015 Sep;396(9-10):991-1002. doi: 10.1515/hsz-2015-0136. Biol Chem. 2015. PMID: 26352204 Review.
Cited by
-
Enhanced imaging of lipid rich nanoparticles embedded in methylcellulose films for transmission electron microscopy using mixtures of heavy metals.Micron. 2017 Aug;99:40-48. doi: 10.1016/j.micron.2017.03.019. Epub 2017 Apr 10. Micron. 2017. PMID: 28419915 Free PMC article.
-
Site-Directed Spin Labeling EPR for Studying Membrane Proteins.Biomed Res Int. 2018 Jan 23;2018:3248289. doi: 10.1155/2018/3248289. eCollection 2018. Biomed Res Int. 2018. PMID: 29607317 Free PMC article. Review.
-
New approaches towards the understanding of integral membrane proteins: A structural perspective on G protein-coupled receptors.Protein Sci. 2017 Aug;26(8):1493-1504. doi: 10.1002/pro.3200. Epub 2017 Jun 7. Protein Sci. 2017. PMID: 28547763 Free PMC article. Review.
-
Determining the Secondary Structure of Membrane Proteins and Peptides Via Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy.Methods Enzymol. 2015;564:289-313. doi: 10.1016/bs.mie.2015.06.037. Epub 2015 Aug 1. Methods Enzymol. 2015. PMID: 26477255 Free PMC article.
-
Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry as a Platform for Characterizing Multimeric Membrane Protein Complexes.J Am Soc Mass Spectrom. 2018 Jan;29(1):183-193. doi: 10.1007/s13361-017-1799-4. Epub 2017 Oct 2. J Am Soc Mass Spectrom. 2018. PMID: 28971338 Free PMC article.
References
-
- Anderson RG. Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. - PubMed
-
- Barrera NP, Di Bartolo N, Booth PJ. Robinson CV. Micelles protect membrane complexes from solution to vacuum. Science. 2008;321:243–246. - PubMed
-
- Barrera NP. Robinson CV. Advances in the mass spectrometry of membrane proteins: from individual proteins to intact complexes. Annu Rev Biochem. 2011;80:247–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

