Milk-based nutraceutical for treating autoimmune arthritis via the stimulation of IL-10- and TGF-β-producing CD39+ regulatory T cells
- PMID: 25629976
- PMCID: PMC4309564
- DOI: 10.1371/journal.pone.0117825
Milk-based nutraceutical for treating autoimmune arthritis via the stimulation of IL-10- and TGF-β-producing CD39+ regulatory T cells
Abstract
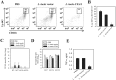
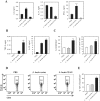
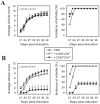
Autoimmune diseases arise from the loss of tolerance to self, and because the etiologies of such diseases are largely unknown, symptomatic treatments rely on anti-inflammatory and analgesic agents. Tolerogenic treatments that can reverse disease are preferred, but again, often thwarted by not knowing the responsible auto-antigens (auto-Ags). Hence, a viable alternative to stimulating regulatory T cells (Tregs) is to induce bystander tolerance. Colonization factor antigen I (CFA/I) has been shown to evoke bystander immunity and to hasten Ag-specific Treg development independent of auto-Ag. To translate in treating human autoimmune diseases, the food-based Lactococcus was engineered to express CFA/I fimbriae, and Lactococcus-CFA/I fermented milk fed to arthritic mice proved highly efficacious. Protection occurred via CD39+ Tregs producing TGF-β and IL-10 to potently suppress TNF-α production and neutrophil influx into the joints. Thus, these data demonstrate the feasibility of oral nutraceuticals for treating arthritis, and potency of protection against arthritis was improved relative to that obtained with Salmonella-CFA/I.
Conflict of interest statement
Figures


Similar articles
-
Segregated regulatory CD39+CD4+ T cell function: TGF-β-producing Foxp3- and IL-10-producing Foxp3+ cells are interdependent for protection against collagen-induced arthritis.J Immunol. 2011 Nov 1;187(9):4654-66. doi: 10.4049/jimmunol.1100530. Epub 2011 Oct 3. J Immunol. 2011. PMID: 21967895 Free PMC article.
-
Oral Escherichia coli colonization factor antigen I fimbriae ameliorate arthritis via IL-35, not IL-27.J Immunol. 2014 Jan 15;192(2):804-16. doi: 10.4049/jimmunol.1302018. Epub 2013 Dec 11. J Immunol. 2014. PMID: 24337375 Free PMC article.
-
IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10.J Immunol. 2010 Jun 15;184(12):7144-53. doi: 10.4049/jimmunol.0902739. Epub 2010 May 10. J Immunol. 2010. PMID: 20483737 Free PMC article.
-
Regulatory T-cell vaccination independent of auto-antigen.Exp Mol Med. 2014 Mar 14;46(3):e82. doi: 10.1038/emm.2014.4. Exp Mol Med. 2014. PMID: 24626168 Free PMC article. Review.
-
Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis.Mod Rheumatol. 2009;19(6):581-9. doi: 10.1007/s10165-009-0210-0. Epub 2009 Aug 21. Mod Rheumatol. 2009. PMID: 19697097 Review.
Cited by
-
Induction of gut regulatory CD39+ T cells by teriflunomide protects against EAE.Neurol Neuroimmunol Neuroinflamm. 2016 Oct 12;3(6):e291. doi: 10.1212/NXI.0000000000000291. eCollection 2016 Dec. Neurol Neuroimmunol Neuroinflamm. 2016. PMID: 27766282 Free PMC article.
-
Oral therapy with colonization factor antigen I prevents development of type 1 diabetes in Non-obese Diabetic mice.Sci Rep. 2020 Apr 9;10(1):6156. doi: 10.1038/s41598-020-62881-4. Sci Rep. 2020. PMID: 32273533 Free PMC article.
-
Regional Differences in the Gut Microbiota and Gut-Associated Immunologic Factors in the Ileum and Cecum of Rats With Collagen-Induced Arthritis.Front Pharmacol. 2020 Nov 24;11:587534. doi: 10.3389/fphar.2020.587534. eCollection 2020. Front Pharmacol. 2020. PMID: 33442384 Free PMC article.
-
Inversed Ratio of CD39/CD73 Expression on γδ T Cells in HIV Versus Healthy Controls Correlates With Immune Activation and Disease Progression.Front Immunol. 2022 Apr 22;13:867167. doi: 10.3389/fimmu.2022.867167. eCollection 2022. Front Immunol. 2022. PMID: 35529864 Free PMC article.
-
Engineering Probiotics for Diabetes Management: Advances, Challenges, and Future Directions in Translational Microbiology.Int J Nanomedicine. 2024 Oct 28;19:10917-10940. doi: 10.2147/IJN.S492651. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39493275 Free PMC article. Review.
References
-
- Cross M, Smith E, Hoy D, Carmona L, Wolfe F et al. (2014) The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis.: 1316–1322. - PubMed
-
- Centers for Disease Control and Prevention (CDC) (2010) Prevalence of doctor diagnosed arthritis and arthritis attributable activity limitation—United States, 2007–2009. MMWR Morb. Mortal. Wkly. Rep. 59: 1261–1265. - PubMed
-
- Scott DL, Wolfe F, Huizinga TW (2012) Rheumatoid arthritis. Lancet 376: 1094–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

