Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation
- PMID: 25629202
- PMCID: PMC4594592
- DOI: 10.1080/15476278.2015.1011921
Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation
Erratum in
-
Erratum.Organogenesis. 2015;11(2):93. doi: 10.1080/15476278.2015.1063374. Organogenesis. 2015. PMID: 26252821 Free PMC article. No abstract available.
Abstract
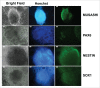
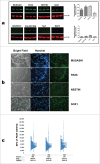
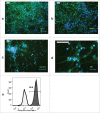
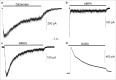
Induced pluripotent stem cell (iPSC)-based technologies offer an unprecedented opportunity to perform high-throughput screening of novel drugs for neurological and neurodegenerative diseases. Such screenings require a robust and scalable method for generating large numbers of mature, differentiated neuronal cells. Currently available methods based on differentiation of embryoid bodies (EBs) or directed differentiation of adherent culture systems are either expensive or are not scalable. We developed a protocol for large-scale generation of neuronal stem cells (NSCs)/early neural progenitor cells (eNPCs) and their differentiation into neurons. Our scalable protocol allows robust and cost-effective generation of NSCs/eNPCs from iPSCs. Following culture in neurobasal medium supplemented with B27 and BDNF, NSCs/eNPCs differentiate predominantly into vesicular glutamate transporter 1 (VGLUT1) positive neurons. Targeted mass spectrometry analysis demonstrates that iPSC-derived neurons express ligand-gated channels and other synaptic proteins and whole-cell patch-clamp experiments indicate that these channels are functional. The robust and cost-effective differentiation protocol described here for large-scale generation of NSCs/eNPCs and their differentiation into neurons paves the way for automated high-throughput screening of drugs for neurological and neurodegenerative diseases.
Keywords: high-throughput screening; induced pluripotent stem cells (iPSCs); neural progenitor cells; neural stem cells; neuronal differentiation.
Figures



Similar articles
-
Generation of induced pluripotent stem cells from rat fibroblasts and optimization of its differentiation into mature functional neurons.J Neurosci Methods. 2024 Jun;406:110114. doi: 10.1016/j.jneumeth.2024.110114. Epub 2024 Mar 24. J Neurosci Methods. 2024. PMID: 38522633
-
Process-based expansion and neural differentiation of human pluripotent stem cells for transplantation and disease modeling.J Neurosci Res. 2013 Oct;91(10):1247-62. doi: 10.1002/jnr.23245. Epub 2013 Jul 26. J Neurosci Res. 2013. PMID: 23893392 Free PMC article.
-
Highly efficient methods to obtain homogeneous dorsal neural progenitor cells from human and mouse embryonic stem cells and induced pluripotent stem cells.Stem Cell Res Ther. 2018 Mar 15;9(1):67. doi: 10.1186/s13287-018-0812-6. Stem Cell Res Ther. 2018. PMID: 29544541 Free PMC article.
-
Applications of induced pluripotent stem cell technologies in spinal cord injury.J Neurochem. 2017 Jun;141(6):848-860. doi: 10.1111/jnc.13986. Epub 2017 Apr 5. J Neurochem. 2017. PMID: 28199003 Review.
-
Modeling neurological diseases using iPSC-derived neural cells : iPSC modeling of neurological diseases.Cell Tissue Res. 2018 Jan;371(1):143-151. doi: 10.1007/s00441-017-2713-x. Epub 2017 Oct 28. Cell Tissue Res. 2018. PMID: 29079884 Free PMC article. Review.
Cited by
-
Population-scale proteome variation in human induced pluripotent stem cells.Elife. 2020 Aug 10;9:e57390. doi: 10.7554/eLife.57390. Elife. 2020. PMID: 32773033 Free PMC article.
-
Neuroregeneration and functional recovery after stroke: advancing neural stem cell therapy toward clinical application.Neural Regen Res. 2021 Jan;16(1):80-92. doi: 10.4103/1673-5374.286955. Neural Regen Res. 2021. PMID: 32788451 Free PMC article.
-
Zika virus vertical transmission induces neuroinflammation and synapse impairment in brain cells derived from children born with Congenital Zika Syndrome.Sci Rep. 2024 Aug 3;14(1):18002. doi: 10.1038/s41598-024-65392-8. Sci Rep. 2024. PMID: 39097642 Free PMC article.
-
Comparison of three cell-based drug screening platforms for HSV-1 infection.Antiviral Res. 2017 Jun;142:136-140. doi: 10.1016/j.antiviral.2017.03.016. Epub 2017 Mar 23. Antiviral Res. 2017. PMID: 28342892 Free PMC article.
-
The hope, hype and obstacles surrounding cell therapy.J Cell Mol Med. 2024 May;28(10):e18359. doi: 10.1111/jcmm.18359. J Cell Mol Med. 2024. PMID: 38770886 Free PMC article. Review.
References
-
- Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development 2013; 140:2457-61; PMID:23715538; http://dx.doi.org/10.1242/dev.092551 - DOI - PubMed
-
- Beevers JE, Caffrey TM, Wade-Martins R. Induced pluripotent stem cell (iPSC)-derived dopaminergic models of Parkinson's disease. Biochem Soc Trans 2013; 41:1503-8; PMID:24256244 - PubMed
-
- Eigentler A, Boesch S, Schneider R, Dechant G, Nat R. Induced pluripotent stem cells from friedreich ataxia patients fail to upregulate frataxin during in vitro differentiation to peripheral sensory neurons. Stem Cells Dev 2013; 22:3271-82; PMID:23879205; http://dx.doi.org/10.1089/scd.2013.0126 - DOI - PubMed
-
- Liu GH, Qu J, Suzuki K, Nivet E, Li M, Montserrat N, Yi F, Xu X, Ruiz S, Zhang W, et al. . Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature 2012; 491:603-7; PMID:23075850; http://dx.doi.org/10.1038/nature11557 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
