Mechanisms of x chromosome dosage compensation
- PMID: 25628761
- PMCID: PMC4303597
- DOI: 10.7150/jgen.10404
Mechanisms of x chromosome dosage compensation
Abstract
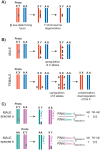
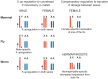
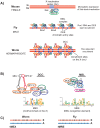
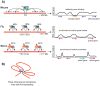
In many animals, males have one X and females have two X chromosomes. The difference in X chromosome dosage between the two sexes is compensated by mechanisms that regulate X chromosome transcription. Recent advances in genomic techniques have provided new insights into the molecular mechanisms of X chromosome dosage compensation. In this review, I summarize our current understanding of dosage imbalance in general, and then review the molecular mechanisms of X chromosome dosage compensation with an emphasis on the parallels and differences between the three well-studied model systems, M. musculus, D. melanogaster and C. elegans.
Keywords: X chromosomes; mechanisms.
Conflict of interest statement
Competing Interests: The author has declared that no competing interest exists.
Figures

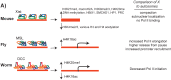
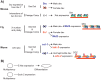
Similar articles
-
Transcriptional modulation of entire chromosomes: dosage compensation.J Genet. 2018 Jun;97(2):357-364. J Genet. 2018. PMID: 29932054 Review.
-
Dosage compensation and sex-specific epigenetic landscape of the X chromosome in the pea aphid.Epigenetics Chromatin. 2017 Jun 15;10:30. doi: 10.1186/s13072-017-0137-1. eCollection 2017. Epigenetics Chromatin. 2017. PMID: 28638443 Free PMC article.
-
Partial dosage compensation in Strepsiptera, a sister group of beetles.Genome Biol Evol. 2015 Jan 18;7(2):591-600. doi: 10.1093/gbe/evv008. Genome Biol Evol. 2015. PMID: 25601100 Free PMC article.
-
Condensin-mediated chromosome organization and gene regulation.Front Genet. 2015 Jan 13;5:473. doi: 10.3389/fgene.2014.00473. eCollection 2014. Front Genet. 2015. PMID: 25628648 Free PMC article. Review.
-
Dosage Compensation of X-Linked Muller Element F Genes but Not X-Linked Transgenes in the Australian Sheep Blowfly.PLoS One. 2015 Oct 27;10(10):e0141544. doi: 10.1371/journal.pone.0141544. eCollection 2015. PLoS One. 2015. PMID: 26506426 Free PMC article.
Cited by
-
SMC-mediated dosage compensation in C. elegans evolved in the presence of an ancestral nematode mechanism.bioRxiv [Preprint]. 2024 May 24:2024.05.21.595224. doi: 10.1101/2024.05.21.595224. bioRxiv. 2024. PMID: 38826443 Free PMC article. Preprint.
-
Neurological risks of COVID-19 in women: the complex immunology underpinning sex differences.Front Immunol. 2023 Nov 14;14:1281310. doi: 10.3389/fimmu.2023.1281310. eCollection 2023. Front Immunol. 2023. PMID: 38035090 Free PMC article.
-
Gene dosage compensation: Origins, criteria to identify compensated genes, and mechanisms including sensor loops as an emerging systems-level property in cancer.Cancer Med. 2023 Dec;12(24):22130-22155. doi: 10.1002/cam4.6719. Epub 2023 Nov 21. Cancer Med. 2023. PMID: 37987212 Free PMC article. Review.
-
Effects of polyploidization and their evolutionary implications are revealed by heritable polyploidy in the haplodiploid wasp Nasonia vitripennis.PLoS One. 2023 Nov 2;18(11):e0288278. doi: 10.1371/journal.pone.0288278. eCollection 2023. PLoS One. 2023. PMID: 37917617 Free PMC article.
-
Master regulator of a mosquito X chromosome discovered.Nature. 2023 Nov;623(7985):34-35. doi: 10.1038/d41586-023-02972-0. Nature. 2023. PMID: 37770657 No abstract available.
References
-
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ. et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–24. doi:10.1126/science.1142210. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

